2.8: Biomolecules
- Page ID
- 28124
There are many interesting biomolecules that are important in biology and biochemistry. Many of them have several organic functional groups that define their specific properties and reactivities.
Glucose
Glucose is initially synthesized by chlorophyll in plants using carbon dioxide from the air and sunlight as an energy source. Glucose is further converted to starch for storage.
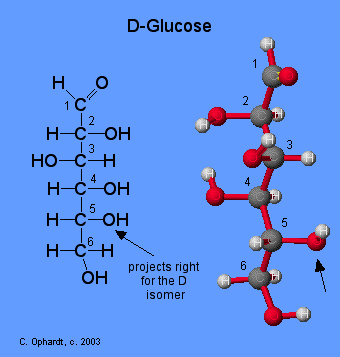
Prostaglandins
Prostaglandins are chemical messengers synthesized in the cells in which their physiological activity is expressed. They are unsaturated fatty acids containing 20 carbon atoms and are synthesized from arachidonic acid—a polyunsaturated fatty acid—when needed by a particular cell. They are called prostaglandins because they were originally isolated from semen found in the prostate gland. It is now known that they are synthesized in nearly all mammalian tissues and affect almost all organs in the body. The five major classes of prostaglandins are designated as PGA, PGB, PGE, PGF, and PGI. Subscripts are attached at the end of these abbreviations to denote the number of double bonds outside the five-carbon ring in a given prostaglandin.
The prostaglandins are among the most potent biological substances known. Slight structural differences give them highly distinct biological effects; however, all prostaglandins exhibit some ability to induce smooth muscle contraction, lower blood pressure, and contribute to the inflammatory response. Aspirin and other nonsteroidal anti-inflammatory agents, such as ibuprofen, obstruct the synthesis of prostaglandins by inhibiting cyclooxygenase, the enzyme needed for the initial step in the conversion of arachidonic acid to prostaglandins.
Their wide range of physiological activity has led to the synthesis of hundreds of prostaglandins and their analogs. Derivatives of PGE2 are now used in the United States to induce labor. Other prostaglandins have been employed clinically to lower or increase blood pressure, inhibit stomach secretions, relieve nasal congestion, relieve asthma, and prevent the formation of blood clots, which are associated with heart attacks and strokes.

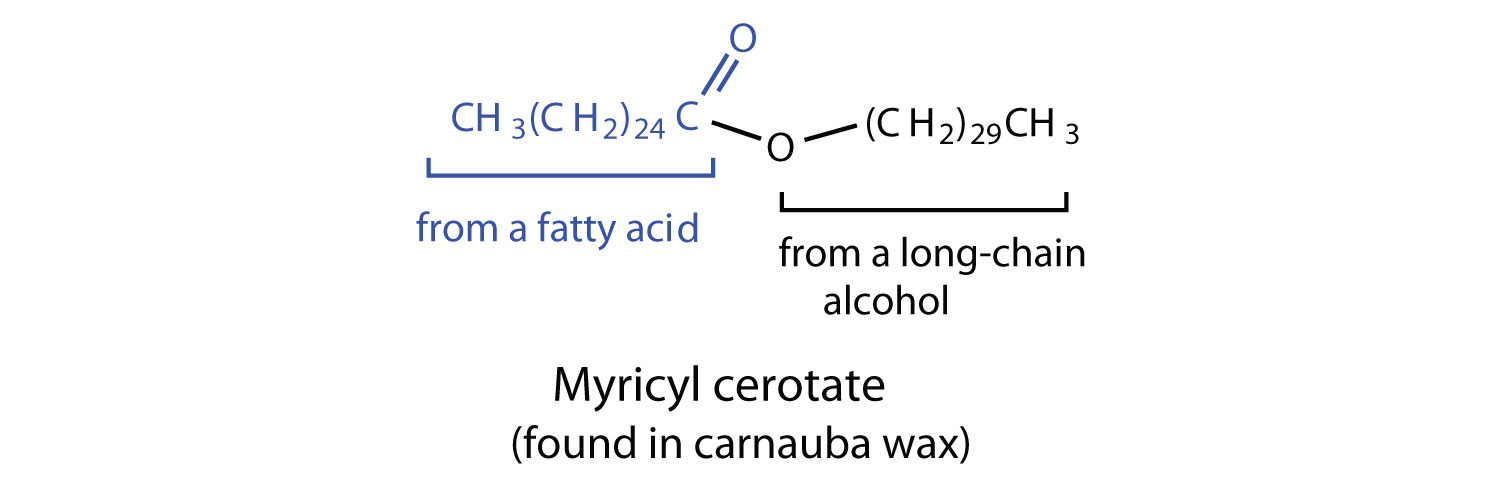
Waxes
Waxes are esters formed from long-chain fatty acids and long-chain alcohols. Most natural waxes are mixtures of such esters. Plant waxes on the surfaces of leaves, stems, flowers, and fruits protect the plant from dehydration and invasion by harmful microorganisms. Carnauba wax, used extensively in floor waxes, automobile waxes, and furniture polish, is largely myricyl cerotate, obtained from the leaves of certain Brazilian palm trees. Animals also produce waxes that serve as protective coatings, keeping the surfaces of feathers, skin, and hair pliable and water repellent. In fact, if the waxy coating on the feathers of a water bird is dissolved as a result of the bird swimming in an oil slick, the feathers become wet and heavy, and the bird, unable to maintain its buoyancy, drowns.
DNA
The secondary structure of DNA is actually very similar to the secondary structure of proteins. The protein single alpha helix structure held together by hydrogen bonds was discovered with the aid of X-ray diffraction studies. The X-ray diffraction patterns for DNA show somewhat similar patterns.
Introduction
In addition, chemical studies by E. Chargaff indicate several important clues about the structure of DNA. In the DNA of all organisms:
- The concentration of adenine equals that of thymine.
- The concentration of guanine equals that of cytosine.
Chargaff's findings clearly indicate that some type of heterocyclic amine base pairing exists in the DNA structure. X-ray diffraction data shows that a repeating helical pattern occurs every 34 Angstrom units with 10 subunits per turn. Each subunit occupies 3.4 Angstrom units which is the same amount of space occupied by a single nucleotide unit. Using Chargaff's information and the X-ray data in conjunction with building actual molecular models, Watson and Crick developed the double helix as a model for DNA.
The double helix in DNA consists of two right-handed polynucleotide chains that are coiled about the same axis. The heterocyclic amine bases project inward toward the center so that the base of one strand interacts or pairs with a base of the other strand. According to the chemical and X-ray data and model building exercises, only specific heterocyclic amine bases may be paired.
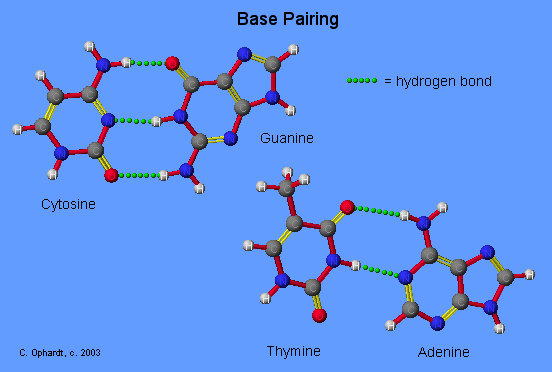
Base Pairing Principle
The Base Pairing Principle is that adenine pairs with thymine (A - T) and guanine pairs with cytosine (G - C)
The base pairing is called complementary because there are specific geometry requirements in the formation of hydrogen bonds between the heterocylic amines. Heterocyclic amine base pairing is an application of the hydrogen bonding principle. In the structures for the complementary base pairs given in the graphic on the left, notice that the thymine - adenine pair interacts through two hydrogen bonds represented as (T=A) and that the cytosine-guanine pair interacts through three hydrogen bonds represented as (C=G).
Although other base pairing-hydrogen bonding combinations may be possible, they are not utilized because the bond distances do not correspond to those given by the base pairs already cited. The diameter of the helix is 20 Angstroms.

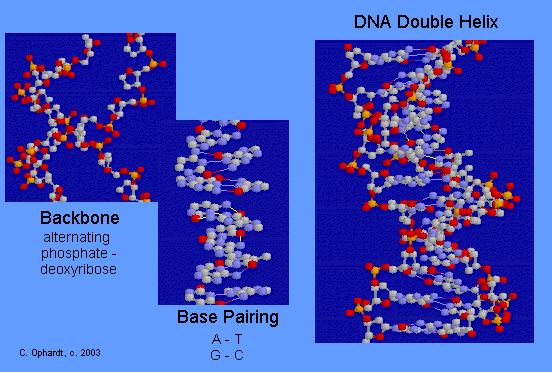
DNA Double Helix
The double-stranded helical model for DNA is shown in the graphic on the left. The easiest way to visualize DNA is as an immensely long rope ladder, twisted into a cork-screw shape. The sides of the ladder are alternating sequences of deoxyribose and phosphate (backbone) while the rungs of the ladder (bases) are made in two parts with each part firmly attached to the side of the ladder. The parts in the rung are heterocyclic amines held in position by hydrogen bonding. Although most DNA exists as open ended double helices, some bacterial DNA has been found as a cyclic helix. Occasionally, DNA has also been found as a single strand.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook


