1.10 Bond Length and Bond Strength
- Page ID
- 407766
The Relationship between Bond Order and Bond Energy
Triple bonds between like atoms are shorter than double bonds, and because more energy is required to completely break all three bonds than to completely break two, a triple bond is also stronger than a double bond. Similarly, double bonds between like atoms are stronger and shorter than single bonds. Bonds of the same order between different atoms show a wide range of bond energies, however. Table 8.6 lists the average values for some commonly encountered bonds. Although the values shown vary widely, we can observe four trends:
Table 1.10.1 Average Bond Energies (kJ/mol) for Commonly Encountered Bonds at 273 K
|
Single Bonds |
|
|
|
|
|
|
|
|
|
Multiple Bonds |
|
|
H–H |
432 |
C–C |
346 |
N–N |
≈167 |
O–O |
≈142 |
F–F |
155 |
C=C |
602 |
|
H–C |
411 |
C–Si |
318 |
N–O |
201 |
O–F |
190 |
F–Cl |
249 |
C≡C |
835 |
|
H–Si |
318 |
C–N |
305 |
N–F |
283 |
O–Cl |
218 |
F–Br |
249 |
C=N |
615 |
|
H–N |
386 |
C–O |
358 |
N–Cl |
313 |
O–Br |
201 |
F–I |
278 |
C≡N |
887 |
|
H–P |
≈322 |
C–S |
272 |
N–Br |
243 |
O–I |
201 |
Cl–Cl |
240 |
C=O |
749 |
|
H–O |
459 |
C–F |
485 |
P–P |
201 |
S–S |
226 |
Cl–Br |
216 |
C≡O |
1072 |
|
H–S |
363 |
C–Cl |
327 |
|
|
S–F |
284 |
Cl–I |
208 |
N=N |
418 |
|
H–F |
565 |
C–Br |
285 |
|
|
S–Cl |
255 |
Br–Br |
190 |
N≡N |
942 |
|
H–Cl |
428 |
C–I |
213 |
|
|
S–Br |
218 |
Br–I |
175 |
N=O |
607 |
|
H–Br |
362 |
Si–Si |
222 |
|
|
|
|
I–I |
149 |
O=O |
494 |
|
H–I |
295 |
Si–O |
452 |
|
|
|
|
|
|
S=O |
532 |
Source: Data from J. E. Huheey, E. A. Keiter, and R. L. Keiter, Inorganic Chemistry, 4th ed. (1993).
- Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Thus an H–F bond is stronger than an H–I bond, H–C is stronger than H–Si, H–N is stronger than H–P, H–O is stronger than H–S, and so forth. The reason for this is that the region of space in which electrons are shared between two atoms becomes proportionally smaller as one of the atoms becomes larger (part (a) in Figure 8.11).
- Bonds between like atoms usually become weaker as we go down a column (important exceptions are noted later). For example, the C–C single bond is stronger than the Si–Si single bond, which is stronger than the Ge–Ge bond, and so forth. As two bonded atoms become larger, the region between them occupied by bonding electrons becomes proportionally smaller, as illustrated in part (b) in Figure 8.11. Noteworthy exceptions are single bonds between the period 2 atoms of groups 15, 16, and 17 (i.e., N, O, F), which are unusually weak compared with single bonds between their larger congeners. It is likely that the N–N, O–O, and F–F single bonds are weaker than might be expected due to strong repulsive interactions between lone pairs of electrons on adjacent atoms. The trend in bond energies for the halogens is therefore
Cl–Cl > Br–Br > F–F > I–I
Similar effects are also seen for the O–O versus S–S and for N–N versus P–P single bonds.
|
Note |
|
Bonds between hydrogen and atoms in a given column in the periodic table are weaker down the column; bonds between like atoms usually become weaker down a column. |
3. Because elements in periods 3 and 4 rarely form multiple bonds with themselves, their multiple bond energies are not accurately known. Nonetheless, they are presumed to be significantly weaker than multiple bonds between lighter atoms of the same families. Compounds containing an Si=Si double bond, for example, have only recently been prepared, whereas compounds containing C=C double bonds are one of the best-studied and most important classes of organic compounds.
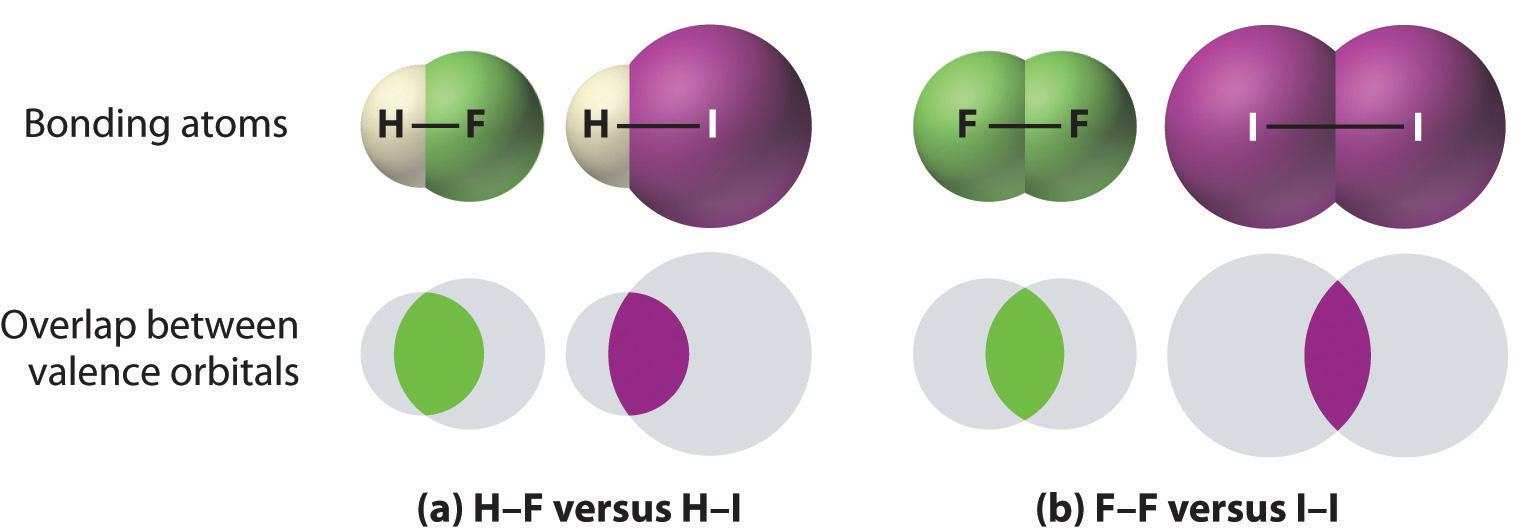
Figure 1.10.1 The Strength of Covalent Bonds Depends on the Overlap between the Valence Orbitals of the Bonded Atoms. The relative sizes of the region of space in which electrons are shared between (a) a hydrogen atom and lighter (smaller) vs. heavier (larger) atoms in the same periodic group; and (b) two lighter versus two heavier atoms in the same group. Although the absolute amount of shared space increases in both cases on going from a light to a heavy atom, the amount of space relative to the size of the bonded atom decreases; that is, the percentage of total orbital volume decreases with increasing size. Hence the strength of the bond decreases.
4. Multiple bonds between carbon, oxygen, or nitrogen and a period 3 element such as phosphorus or sulfur tend to be unusually strong. In fact, multiple bonds of this type dominate the chemistry of the period 3 elements of groups 15 and 16. Multiple bonds to phosphorus or sulfur occur as a result of d-orbital interactions, as we discussed for the SO42− ion in Section 8.6. In contrast, silicon in group 14 has little tendency to form discrete silicon–oxygen double bonds. Consequently, SiO2 has a three-dimensional network structure in which each silicon atom forms four Si–O single bonds, which makes the physical and chemical properties of SiO2 very different from those of CO2.
|
Note |
|
Bond strengths increase as bond order increases, while bond distances decrease. |
Table: Average bond energies:
|
Bond |
(kJ/mol) |
|
C-F |
485 |
|
C-Cl |
328 |
|
C-Br |
276 |
|
C-I |
240 |
|
|
|
|
C-C |
348 |
|
C-N |
293 |
|
C-O |
358 |
|
C-F |
485 |
|
|
|
|
C-C |
348 |
|
C=C |
614 |
|
C=C |
839 |
Contributors
- Kim Song (UCD), Donald Le (UCD)

