22.2 General Nucleophilic Acyl Substitution Reaction
- Page ID
- 28412
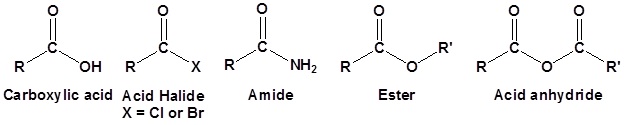
Carboxylic acid derivatives are functional groups whose chemistry is closely related. The main difference is the presence of an electronegative substituent that can act as a leaving group during a nucleophile substitution reaction. Although there are many types of carboxylic acid derivatives known, this article focuses on four: acid halides, acid anhydrides, esters, and amides.
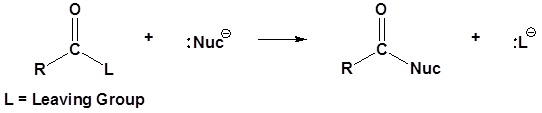
General reaction
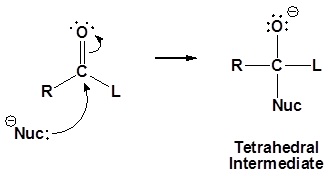
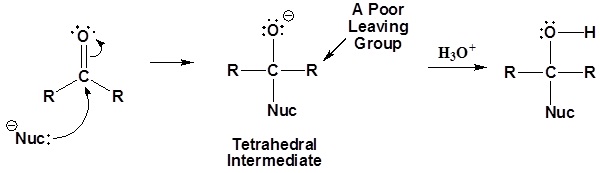
General mechanism
1) Nucleophilic attack on the carbonyl
2) Leaving group is removed
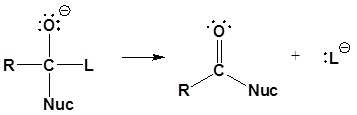
Although aldehydes and ketones also contain carbonyls, their chemistry is distinctly different because they do not contain suitable leaving groups. Once a tetrahedral intermediate is formed, aldehydes and ketones cannot reform their carbonyls. Because of this, aldehydes and ketones typically undergo nucleophilic additions and not substitutions.
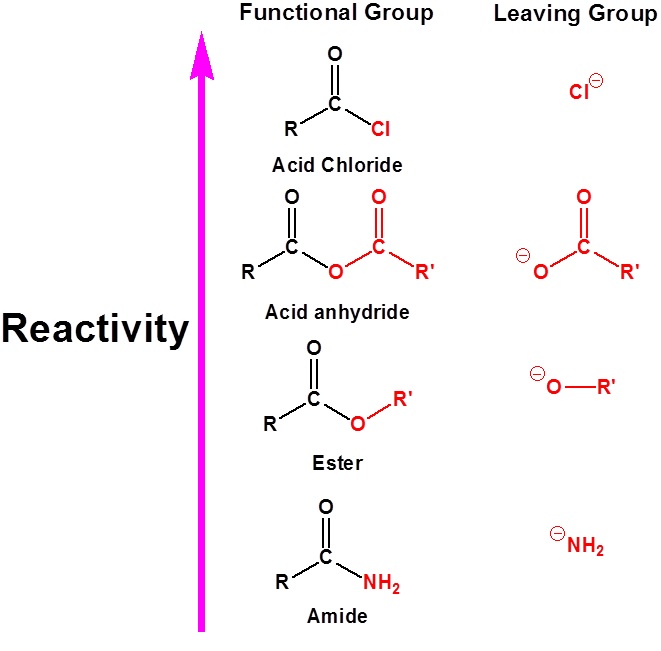
The relative reactivity of carboxylic acid derivatives toward nucleophile substitutions is related to the electronegative leaving group’s ability to activate the carbonyl. The more electronegative leaving groups withdraw electron density from the carbonyl, thereby increasing its electrophilicity.
Contributors
Prof. Steven Farmer (Sonoma State University)


