16.4 The Resonance Hybrid
- Page ID
- 28325
Resonance contributors for the carboxylate group
The convention of drawing two or more resonance contributors to approximate a single structure may seem a bit clumsy to you at this point, but as you gain experience you will see that the practice is actually very useful when discussing the manner in which many functional groups react. Let’s next consider the carboxylate ion (the conjugate base of a carboxylic acid). As our example, we will use formate, the simplest possible carboxylate-containing molecule. The conjugate acid of formate is formic acid, which causes the painful sting you felt if you have ever been bitten by an ant.
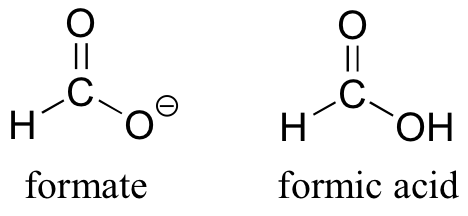
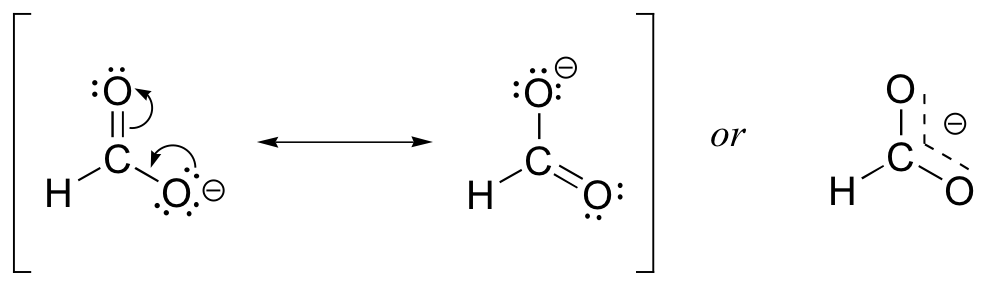
Usually, you will see carboxylate groups drawn with one carbon-oxygen double bond and one carbon-oxygen single bond, with a negative formal charge located on the single-bonded oxygen. In actuality, however, the two carbon-oxygen bonds are the same length, and although there is indeed an overall negative formal charge on the group, it is shared equally between the two oxygens. Therefore, the carboxylate can be more accurately depicted by a pair of resonance contributors. Alternatively, a single structure can be used, with a dashed line depicting the resonance-delocalized π bond and the negative charge located in between the two oxygens.
Let’s see if we can correlate these drawing conventions to a valence bond theory picture of the bonding in a carboxylate group. We know that the carbon must be sp2-hybridized, (the bond angles are close to 120˚, and the molecule is planar), and we will treat both oxygens as being sp2-hybridized as well. Both carbon-oxygen sbonds, then, are formed from the overlap of carbon sp2 orbitals and oxygen sp2 orbitals.
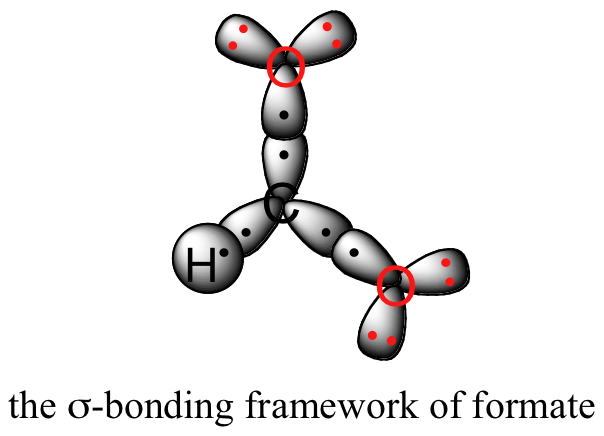
In addition, the carbon and both oxygens each have an unhybridized 2pz orbital situated perpendicular to the plane of the sigma bonds. These three 2pz orbitals are parallel to each other, and can overlap in a side-by-side fashion to form a delocalized pi bond.
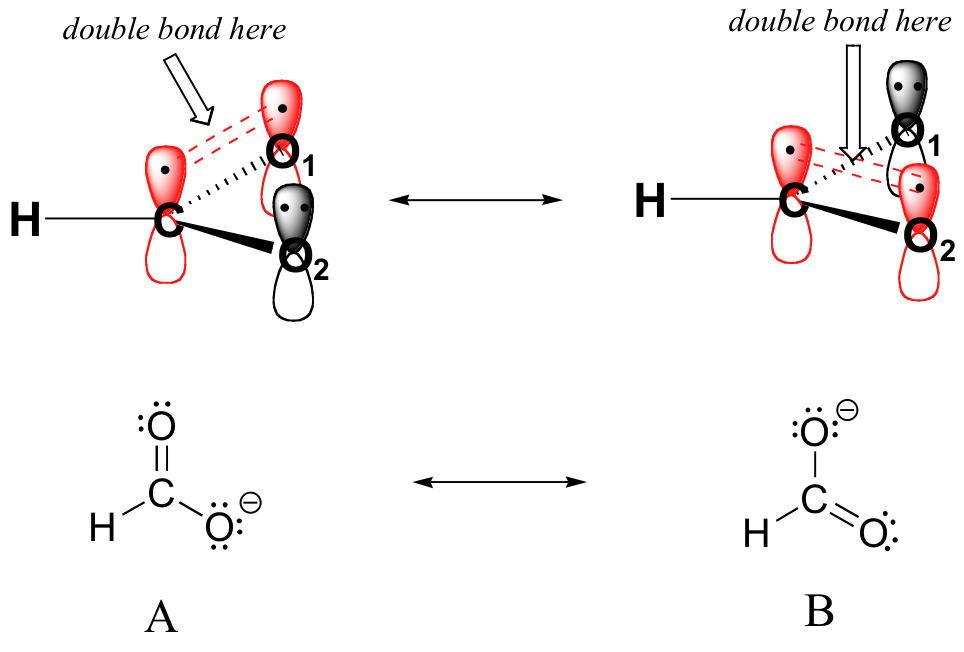
Resonance contributor A shows oxygen #1 sharing a pair of electrons with carbon in a pi bond, and oxygen #2 holding a lone pair of electrons in its 2pz orbital. Resonance contributor B, on the other hand, shows oxygen #2 participating in the pi bond with carbon, and oxygen #1 holding a lone pair in its 2pz orbital. Overall, the situation is one of three parallel, overlapping 2pz orbitals sharing four delocalized pi electrons. Because there is one more electron than there are 2pz orbitals, the system has an overall charge of –1. This is the kind of 3D picture that resonance contributors are used to approximate, and once you get some practice you should be able to quickly visualize overlapping 2pz orbitals and delocalized pi electrons whenever you see resonance structures being used. In this text, carboxylate groups will usually be drawn showing only one resonance contributor for the sake of simplicity, but you should always keep in mind that the two C-O bonds are equal, and that the negative charge is delocalized to both oxygens.
Major vs minor resonance contributors
Different resonance contributors do not always make the same contribution to the overall structure of the hybrid. If one resonance structure is more stable (lower in energy) than another, then the first will come closer to depicting the ‘real’ (hybrid) structure than the second. In the case of carboxylates, contributors A and B are equivalent to each other in terms of their relative stability and therefore their relative contribution to the hybrid structure. However, there is a third resonance contributor ‘C’ that we have not considered yet, in which the carbon bears a positive formal charge and both oxygens are single-bonded and bear negative charges.
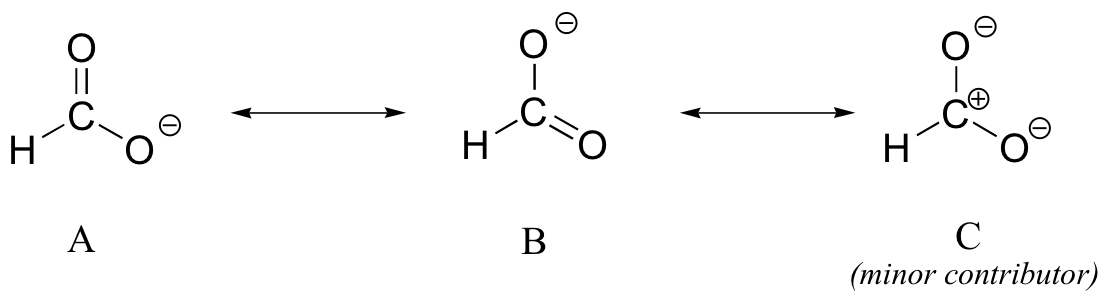
Structure C is relatively less stable, and therefore makes a less important contribution to the overall structure of the hybrid relative to A and B.
How do we know that structure C is the less stable, and thus the ‘minor’ contributor? There are four basic rules which you need to learn in order to evaluate the relative stability of different resonance contributors. We will number them 5-8 so that they may be added to in the 'rules for resonance' list from section 2.2C.
5) The carbon in contributor C does not have an octet – in general, resonance contributors in which a carbon does not fulfill the octet rule are relatively less important.
6) In structure C, a separation of charge has been introduced that is not present in A or B. In general, resonance contributors in which there is a greater separation of charge are relatively less important.
7) In structure C, there are no double bonds. In general, a resonance structure with a lower number of multiple bonds is relatively less important.
There is one more important rule that does not apply to this particular example, but we will list it here in the interest of completeness (we will soon see an actual example).
8) The resonance contributor in which a negative formal charge is located on a more electronegative atom (such as oxygen or nitrogen) is more stable than one in which the negative charge is located on a less electronegative atom (such as carbon).
When discussing other examples of resonance contributors, we will often see cases where one form is less stable – but these minor resonance contributors can still be very relevant in explaining properties of structure or reactivity, and should not be disregarded.


