16.3 Common Examples of Resonance
- Page ID
- 28324
1) Three atoms in a A=B-C where C is an atom with a p orbital.
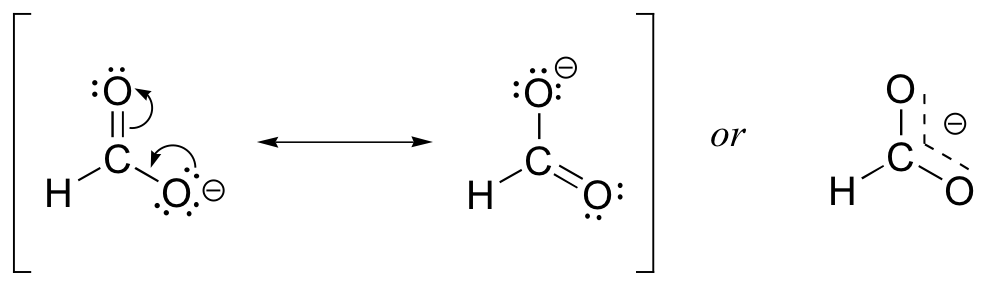
There are two major resonance structures possible. The two structures differ in the location of the double bond. The anion, cation, or radical is stabilized by delocaliztion.
Examples
Carboxylate Anion
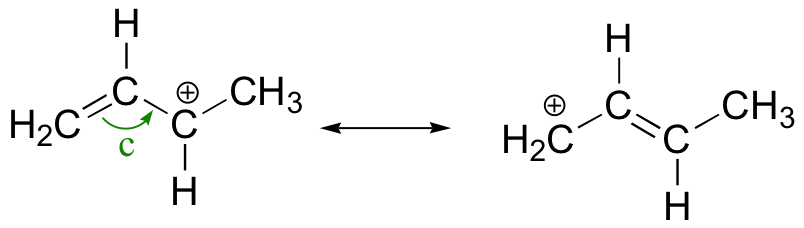
Allylic carbocation
Allyic radical
2) Conjugated double bonds
The benzene ring has two resonance structures which can be drawn by moving elections in a cyclic manner.
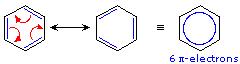
Conjugated double bonds contain multiple resonance structures.
3) Cations adjacent of an atom with lone pair electons.
Because heteroatoms such as oxygen and nitrogen are more electronegative than carbon, you might expect that they would by definition be electron withdrawing groups that destabilize carbocations. In fact, the opposite is often true: if the oxygen or nitrogen atom is in the correct position, the overall effect is carbocation stabilization. This is due to the fact that although these heteroatoms are electron withdrawing groups by induction, they are electron donating groups by resonance, and it is this resonance effect which is more powerful. (We previously encountered this same idea when considering the relative acidity and basicity of phenols and aromatic amines in section 7.4). Consider the two pairs of carbocation species below:
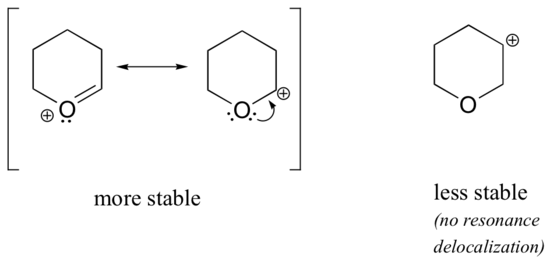
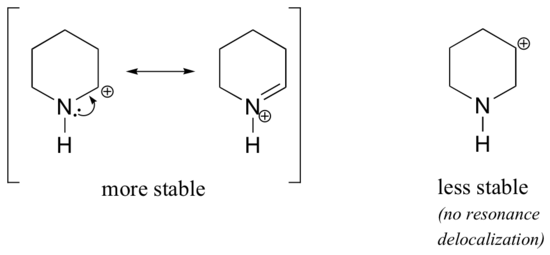
In the more stable carbocations, the heteroatom acts as an electron donating group by resonance: in effect, the lone pair on the heteroatom is available to delocalize the positive charge. In the less stable carbocations the positively-charged carbon is more than one bond away from the heteroatom, and thus no resonance effects are possible. In fact, in these carbocation species the heteroatoms actually destabilize the positive charge, because they are electron withdrawing by induction
4) Double bonds with one atom more electronegative that the other
Multiple resonance structures are possible which causes a charge separation in the molecule.
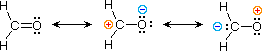
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)
- Layne A. Morsch (University of Illinois Springfield)


