9.2: Structure and Bonding of Alcohols, Ethers and Epoxides
- Page ID
- 28196
Structure of Alcohols
The structure of an alcohol resembles that of water. With both alcohol and water, the bond angles reflect the effect of electron repulsion and increasing steric bulk of the substituents on the central oxygen atom. The electronegativity of oxygen contributes to the unsymmetrical distribution of charge, creating a partial positive charge on hydrogen and a partial negative charge on oxygen. This uneven distribution of electron density in the O-H bond creates a dipole. In addition, because of the high electronegativity of oxygen relative to that of carbon, the O-H bond is shorter (1.41 Å for C-O vs. 1.51 Å for C-C) and stronger (ΔHoO-H = 104 kcal mol-1 for C-O vs. ΔHoC-H = 98 kcal mol-1 for C-C).
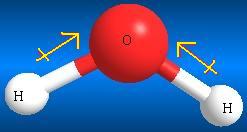
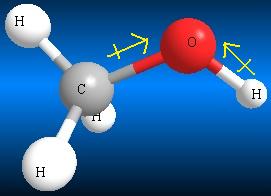
Water Methanol
Structure of Epoxides
Epoxides (also known as oxiranes) are three-membered ring structures in which one of the vertices is an oxygen and the other two are carbons.
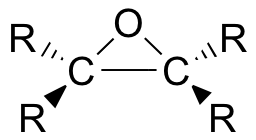
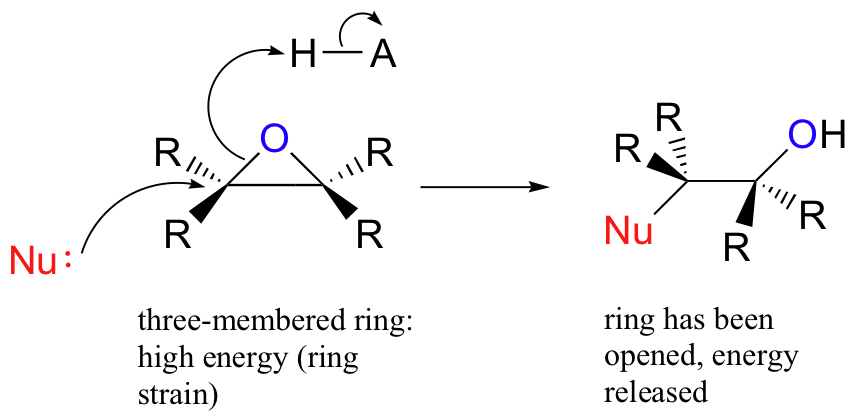
The carbons in an epoxide group are very reactive electrophiles, due in large part to the fact that substantial ring strain is relieved when the ring opens upon nucleophilic attack.
Structure of Ethers
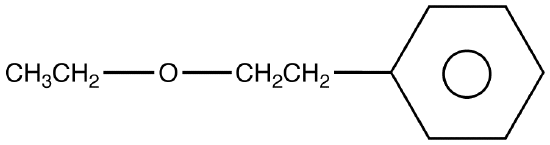
Ethers are a class of organic compounds that contain an oxygen between two alkyl groups. They have the formula R-O-R', with R's being the alkyl groups. these compounds are used in dye, perfumes, oils, waxes and industrial use. Ethers are named as alkoxyalkanes.An aliphatic ether is an ether in the molecule of which there are no aryl groups on the ether group.
eg:

An ether molecule may contain aryl groups, nevertheless, be an aliphatic ether.
eg:
- Jaspreet Dheri
- Gamini Gunawardena from the OChemPal site (Utah Valley University)
- Layne A. Morsch (University of Illinois Springfield)


