7.5: The Polar Carbon–Halogen Bond
- Page ID
- 28168
Halogens and the Character of the Carbon-Halogen Bond
With respect to electronegativity, halogens are more electronegative than carbons. This results in a carbon-halogen bond that is polarized. As shown in the image below, carbon atom has a partial positive charge, while the halogen has a partial negative charge.
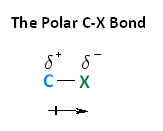
The following image shows the relationship between the halogens and electronegativity. Notice, as we move up the periodic table from iodine to fluorine, electronegativity increases.
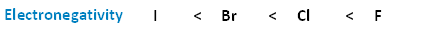
The following image shows the relationships between bond length, bond strength, and molecular size. As we progress down the periodic table from fluorine to iodine, molecular size increases. As a result, we also see an increase in bond length. Conversely, as molecular size increases and we get longer bonds, the strength of those bonds decreases.

The influence of bond polarity
Of the four halogens, fluorine is the most electronegative and iodine the least. That means that the electron pair in the carbon-fluorine bond will be dragged most towards the halogen end. Looking at the methyl halides as simple examples:

The electronegativities of carbon and iodine are equal and so there will be no separation of charge on the bond.
One of the important set of reactions of alkyl halides involves replacing the halogen by something else - substitution reactions. These reactions involve either:
- the carbon-halogen bond breaking to give positive and negative ions. The ion with the positively charged carbon atom then reacts with something either fully or slightly negatively charged.
- something either fully or negatively charged attracted to the slightly positive carbon atom and pushing off the halogen atom.
You might have thought that either of these would be more effective in the case of the carbon-fluorine bond with the quite large amounts of positive and negative charge already present. But that's not so - quite the opposite is true! The thing that governs the reactivity is the strength of the bonds which have to be broken. If is difficult to break a carbon-fluorine bond, but easy to break a carbon-iodine one.
Contributors
- Rachael Curtis (UC Davis)
Jim Clark (Chemguide.co.uk)


