4.7: R and S Assignments in Compounds with Two or More Stereogenic Centers
- Page ID
- 28148
The Chinese shrub Ma Huang (Ephedra vulgaris) contains two physiologically active compounds ephedrine and pseudoephedrine. Both compounds are stereoisomers of 2-methylamino-1-phenyl-1-propanol, and both are optically active, one being levorotatory and the other dextrorotatory. Since the properties of these compounds (see below) are significantly different, they cannot be enantiomers. How, then, are we to classify these isomers and others like them?
- Ephedrine: m.p. 35 - 40 º C, [α]D = –41º, moderate water solubility [this isomer may be referred to as (–)-ephedrine]
- Pseudoephedrine: m.p. 119 º C, [α]D = +52º, relatively insoluble in water [this isomer may be referred to as (+)-pseudoephedrine]
Since these two compounds are optically active, each must have an enantiomer. Although these missing stereoisomers were not present in the natural source, they have been prepared synthetically and have the expected identical physical properties and opposite-sign specific rotations with those listed above. The structural formula of 2-methylamino-1-phenylpropanol has two stereogenic carbons (#1 & #2). Each may assume an R or S configuration, so there are four stereoisomeric combinations possible. These are shown in the following illustration, together with the assignments that have been made on the basis of chemical interconversions
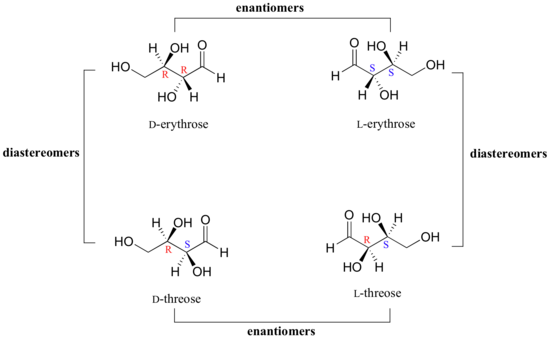
We turn our attention next to molecules which have more than one stereocenter. We will start with a common four-carbon sugar called D-erythrose.
A note on sugar nomenclature: biochemists use a special system to refer to the stereochemistry of sugar molecules, employing names of historical origin in addition to the designators 'D' and 'L'. You will learn about this system if you take a biochemistry class. We will use the D/L designations here to refer to different sugars, but we won't worry about learning the system.
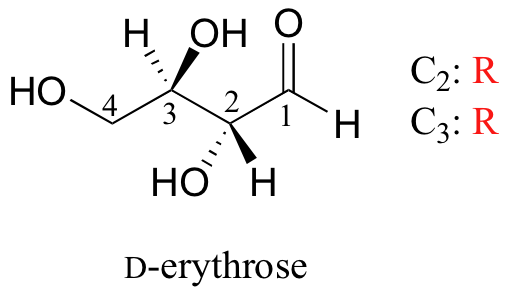
As you can see, D-erythrose is a chiral molecule: C2 and C3 are stereocenters, both of which have the R configuration. In addition, you should make a model to convince yourself that it is impossible to find a plane of symmetry through the molecule, regardless of the conformation. Does D-erythrose have an enantiomer? Of course it does – if it is a chiral molecule, it must. The enantiomer of erythrose is its mirror image, and is named L-erythrose (once again, you should use models to convince yourself that these mirror images of erythrose are not superimposable).
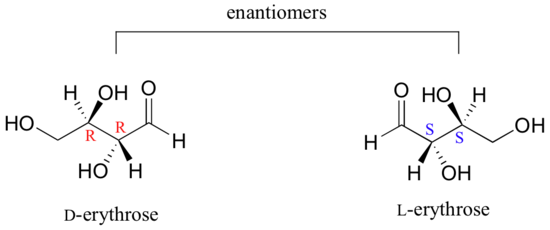
Notice that both chiral centers in L-erythrose both have the S configuration. In a pair of enantiomers, all of the chiral centers are of the opposite configuration.
What happens if we draw a stereoisomer of erythrose in which the configuration is S at C2 and R at C3? This stereoisomer, which is a sugar called D-threose, is not a mirror image of erythrose. D-threose is a diastereomer of both D-erythrose and L-erythrose.
The definition of diastereomers is simple: if two molecules are stereoisomers (same molecular formula, same connectivity, different arrangement of atoms in space) but are not enantiomers, then they are diastereomers by default. In practical terms, this means that at least one - but not all - of the chiral centers are opposite in a pair of diastereomers. By definition, two molecules that are diastereomers are not mirror images of each other.
L-threose, the enantiomer of D-threose, has the R configuration at C2 and the S configuration at C3. L-threose is a diastereomer of both erythrose enantiomers.
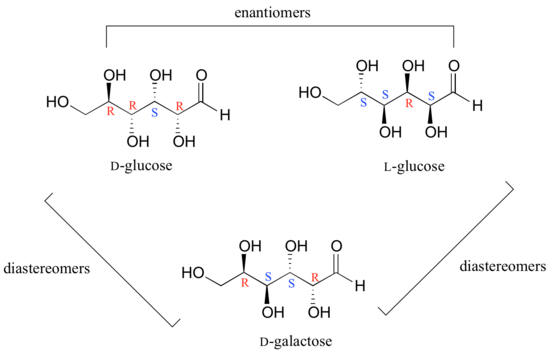
In general, a structure with n stereocenters will have 2n different stereoisomers. (We are not considering, for the time being, the stereochemistry of double bonds – that will come later). For example, let's consider the glucose molecule in its open-chain form (recall that many sugar molecules can exist in either an open-chain or a cyclic form). There are two enantiomers of glucose, called D-glucose and L-glucose. The D-enantiomer is the common sugar that our bodies use for energy. It has n = 4 stereocenters, so therefore there are 2n = 24 = 16 possible stereoisomers (including D-glucose itself).
In L-glucose, all of the stereocenters are inverted relative to D-glucose. That leaves 14 diastereomers of D-glucose: these are molecules in which at least one, but not all, of the stereocenters are inverted relative to D-glucose. One of these 14 diastereomers, a sugar called D-galactose, is shown above: in D-galactose, one of four stereocenters is inverted relative to D-glucose. Diastereomers which differ in only one stereocenter (out of two or more) are called epimers. D-glucose and D-galactose can therefore be refered to as epimers as well as diastereomers.
Exercise 5.9.2
Draw the structure of two more diastereomers of D-glucose. One should be an epimer.
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry