3.1: Alkanes—An Introduction
- Page ID
- 28125
Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes have the general formula \(C_nH_{2n+2}\) and can be subdivided into the following three groups: the linear straight-chain alkanes, branched alkanes, and cycloalkanes. Alkanes are also saturated hydrocarbons.
Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. Cycloalkanes are also saturated, meaning that all of the carbons atoms that make up the ring are single bonded to other atoms (no double or triple bonds). There are also polycyclic alkanes, which are molecules that contain two or more cycloalkanes that are joined, forming multiple rings.
This is an introductory page about alkanes, such as methane, ethane, propane, butane and the remainder of the common alkanes. This page addresses their formulae and isomerism, their physical properties, and an introduction to their chemical reactivity.
Molecular Formulas
Alkanes are the simplest family of hydrocarbons - compounds containing carbon and hydrogen only. Alkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds. The first six alkanes are as follows:
| methane | CH4 |
| ethane | C2H6 |
| propane | C3H8 |
| butane | C4H10 |
| pentane | C5H12 |
| hexane | C6H14 |
You can work out the formula of any of the alkanes using the general formula CnH2n+2
Isomerism
All of the alkanes containing 4 or more carbon atoms show structural isomerism, meaning that there are two or more different structural formulae that you can draw for each molecular formula.
Example 4.1.1: Butane or MethylPropane
C4H10 could be either of these two different molecules:
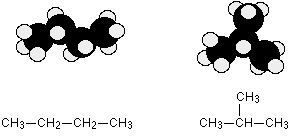
These are named butane and 2-methylpropane, respectively.
What is structural isomerism?
Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers. Both are butane.
There are also endless other possible ways that this molecule could twist itself. There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
In structural isomerism, the atoms are arranged in a completely different order. This is easier to see with specific examples. What follows looks at some of the ways that structural isomers can arise. The names of the various forms of structural isomerism probably don't matter all that much, but you must be aware of the different possibilities when you come to draw isomers.
Chain isomerism
These isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a "straight chain" whereas in the other the chain is branched.
Be careful not to draw "false" isomers which are just twisted versions of the original molecule. For example, this structure is just the straight chain version of butane rotated about the central carbon-carbon bond.
You could easily see this with a model. This is the example we've already used at the top of this page.
Example 4.1.2: Chain Isomers in Pentane
Pentane, C5H12, has three chain isomers. If you think you can find any others, they are simply twisted versions of the ones below. If in doubt make some models.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Prof. Steven Farmer (Sonoma State University)
- Layne Morsch (University of Illinois Springfield)


