5.6: Lewis Acids and Bases
- Page ID
- 28115
According to the Lewis theory, an acid is an electron pair acceptor, and a base is an electron pair donor. Lewis bases are also Brønsted bases; however, many Lewis acids, such as BF3, AlCl3 and Mg2+, are not Brønsted acids. The product of a Lewis acid-base reaction, is a neutral, dipolar or charged complex, which may be a stable covalent molecule. As shown at the top of the following drawing, coordinate covalent bonding of a phosphorous Lewis base to a boron Lewis acid creates a complex in which the formal charge of boron is negative and that of phosphorous is positive. In this complex, boron acquires a neon valence shell configuration and phosphorous an argon configuration. If the substituents (R) on these atoms are not large, the complex will be favored at equilibrium. However, steric hindrance of bulky substituents may prohibit complex formation. The resulting mixture of non-bonded Lewis acid/base pairs has been termed "frustrated", and exhibits unusual chemical behavior.
Two examples of Lewis acid-base equilibria that play a role in chemical reactions are shown in equations 1 & 2 below.
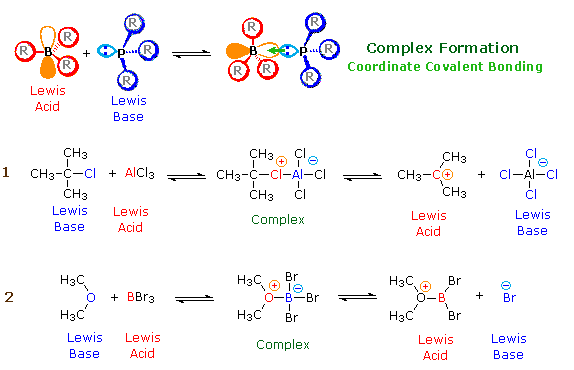
In the first example, an electron deficient aluminum atom bonds to a covalent chlorine atom by sharing one of its non-bonding valence electron pairs, and thus achieves an argon-like valence shell octet. Because this sharing is unilateral (chlorine contributes both electrons), both the aluminum and the chlorine have formal charges, as shown. If the carbon chlorine bond in this complex breaks with both the bonding electrons remaining with the more electronegative atom (chlorine), the carbon assumes a positive charge. We refer to such carbon species as carbocations. Carbocations are also Lewis acids, as the reverse reaction demonstrates.
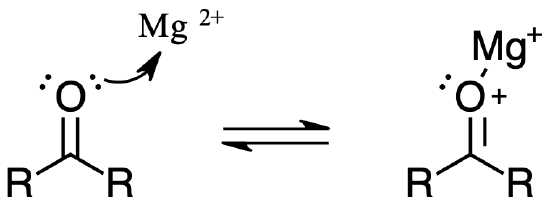
Many carbocations (but not all) may also function as Brønsted acids. Equation 3 illustrates this dual behavior; the Lewis acidic site is colored red and three of the nine acidic hydrogen atoms are colored orange. In its Brønsted acid role the carbocation donates a proton to the base (hydroxide anion), and is converted to a stable neutral molecule having a carbon-carbon double bond.
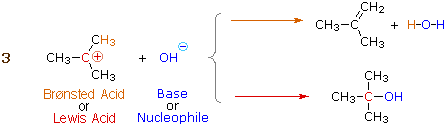
The interaction between a magnesium cation (Mg+2) and a carbonyl oxygen is a common example of a Lewis acid-base reaction. The carbonyl oxygen (the Lewis base) donates a pair of electrons to the magnesium cation (the Lewis acid).
As we will see, we begin the study of reactions involving carbonyl groups, this interaction has the very important effect of increasing the polarity of the carbon-oxygen double bond.
The Brønsted-Lowry equivalent of the reaction above is simply protonation of the carbonyl group. This, too, has the effect of increasing the polarity of the carbonyl double bond.
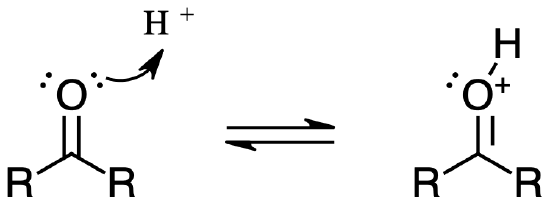
While it is important to be familiar with the Lewis definition, the focus throughout the remainder of this chapter will be on acid-base reactions of the Brønsted-Lowry type, where an actual proton transfer event takes place.
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Layne A. Morsch (University of Illinois Springfield)


