18.4 Aromatic Nitration and Sulfonation
- Page ID
- 28351
Nitration and sulfonation of benzene are two examples of electrophilic aromatic substitution. The nitronium ion (NO2+) and sulfur trioxide (SO3) are the electrophiles and individually react with benzene to give nitrobenzene and benzenesulfonic acid respectively.
Nitration of Benzene
The source of the nitronium ion is through the protonation of nitric acid by sulfuric acid, which causes the loss of a water molecule and formation of a nitronium ion.
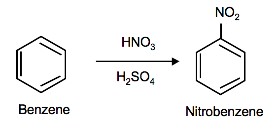
Sulfuric Acid Activation of Nitric Acid
The first step in the nitration of benzene is to activate HNO3with sulfuric acid to produce a stronger electrophile, the nitronium ion.
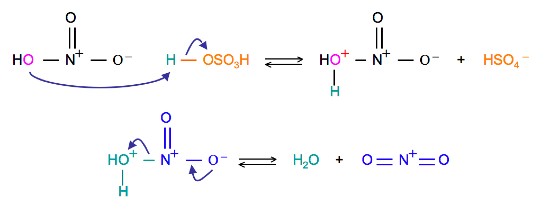
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce Nitrobenzene.
Mechanism
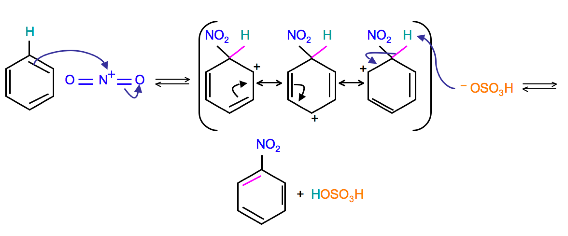
(Resonance forms of the intermediate can be seen in the generalized electrophilic aromatic substitution)
Sulfonation of Benzene
Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.
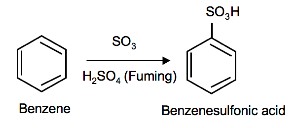
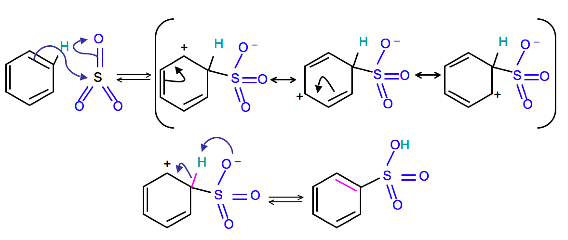
Mechanism
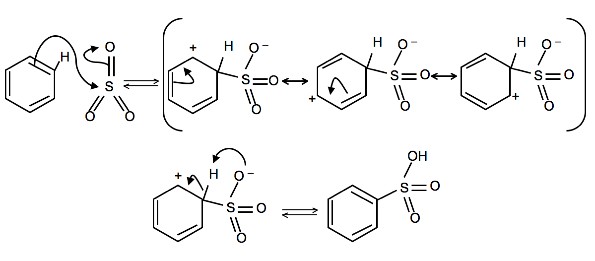
To produce benzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added. Fuming sulfuric acid, also refered to as oleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid. The sulfur in sulfur trioxide is electrophilic because the oxygens pull electrons away from it because oxygen is very electronegative. The benzene attacks the sulfur (and subsequent proton transfers occur) to produce benzenesulfonic acid.
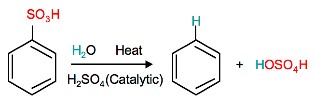
Reverse Sulfonation
Sulfonation of benzene is a reversible reaction. Sulfur trioxide readily reacts with water to produce sulfuric acid and heat. Therefore, by adding heat to benzenesulfonic acid in diluted aqueous sulfuric acid the reaction is reversed.
Further Applications of Nitration and Sulfonation
Nitration is used to add nitrogen to a benzene ring, which can be used further in substitution reactions. The nitro group acts as a ring deactivator. Having nitrogen present in a ring is very useful because it can be used as a directing group as well as a masked amino group. The products of aromatic nitrations are very important intermediates in industrial chemistry.
Because sulfonation is a reversible reaction, it can also be used in further substitution reactions in the form of a directing blocking group because it can be easily removed. The sulfonic group blocks the carbon from being attacked by other substituents and after the reaction is completed it can be removed by reverse sulfonation. Benzenesulfonic acids are also used in the synthesis of detergents, dyes, and sulfa drugs. Bezenesulfonyl Chloride is a precursor to sulfonamides, which are used in chemotherapy.
Outside Links
Aromatic Sulfonation
- Wikipedia: http://en.wikipedia.org/wiki/Aromatic_sulfonation
- Video: http://www.youtube.com/watch?v=s1qJ1...eature=related
- Interactive 3D Reaction: http://www.chemtube3d.com/Electrophi...20benzene.html
Aromatic Nitration
- Wikipedia: http://en.wikipedia.org/wiki/Nitration
- Video: http://www.youtube.com/watch?v=i7ucl...eature=related
- Interactive 3D Reaction: http://www.chemtube3d.com/Electrophi...20benzene.html
Problems
1. What is/are the required reagent(s)for the following reaction:
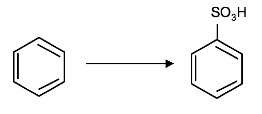
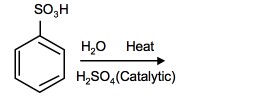
2. What is the product of the following reaction:
3. Why is it important that the nitration of benzene by nitric acid occurs in sulfuric acid?
4. Write a detailed mechanism for the sulfonation of benzene, including all resonance forms.
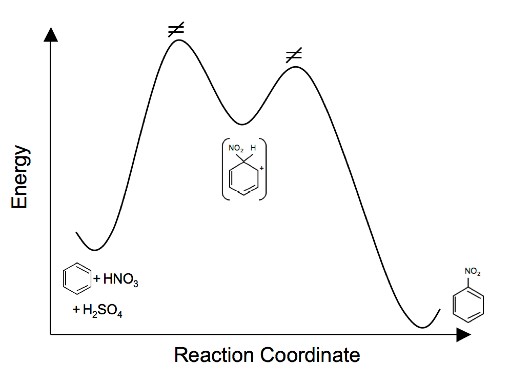
5. Draw an energy diagram for the nitration of benzene. Draw the intermediates, starting materials, and products. Label the transition states. (For questions 1 and 2 see Electrophilic Aromatic Substitution for hints)
For other problems involving Electrophilic Aromatic Substitution and similar reactions see:
Solutions
1. SO3 and H2SO4 (fuming)
2.
3. Sulfuric acid is needed in order for a good electrophile to form. Sulfuric acid protonates nitric acid to form the nitronium ion (water molecule is lost). The nitronium ion is a very good electrophile and is open to attack by benzene. Without sulfuric acid the reaction would not occur.
4.
5.
References
- Laali, Kenneth K., and Volkar J. Gettwert. “Electrophilic Nitration of Aromatics in Ionic Liquid Solvents.” The Journal of Organic Chemistry 66 (Dec. 2000): 35-40. American Chemical Society.
- Malhotra, Ripudaman, Subhash C. Narang, and George A. Olah. Nitration: Methods and Mechanisms. New York: VCH Publishers, Inc., 1989.
- Sauls, Thomas W., Walter H. Rueggeberg, and Samuel L. Norwood. “On the Mechanism of Sulfonation of the Aromatic Nucleus and Sulfone Formation.” The Journal of Organic Chemistry 66 (1955): 455-465. American Chemical Society.
- Vollhardt, Peter. Organic Chemistry : Structure and Function. 5th ed. Boston: W. H. Freeman & Company, 2007.


