3.11: Quiz 2B
- Page ID
- 19094
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
- Clearly indicate true (T) or false (F) for the following statements (2 pts each):
| _____ | Cyclopropane has more ring strain than cyclohexane. |
| _____ | A pi bond is stronger than a sigma bond. |
| _____ | The staggered conformation of ethane is more stable than the eclipsed form of ethane because there is less strain and electron repulsion. |
| _____ | Propyl ethyl ether is a molecule with 6 carbon atoms. |
- Multiple Choice: (2 pts each)
- Which of the following statements about propene, CH3CH=CH2, is correct?
 All nine atoms lie in the same plane
All nine atoms lie in the same plane- All three carbon atoms lie in the same plane
- The compound has a cis and trans isomer
- All the carbon atoms are sp2 hybridized
- Which of the following is the strongest interaction?
 hydrogen bonding
hydrogen bonding - induced dipole-induced dipole interactions
- dipole-dipole interactions
- a covalent bond
- Van der Waals
 Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
- Which of the following statements about propene, CH3CH=CH2, is correct?
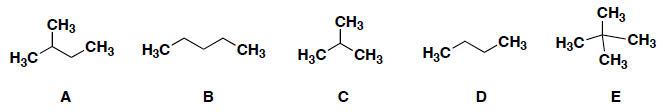
 Which of the following has the greatest solubility in an organic solvent such as hexanes?
Which of the following has the greatest solubility in an organic solvent such as hexanes?
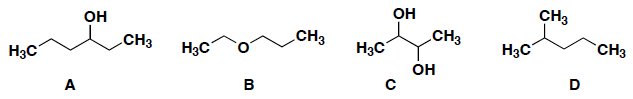
 Which of the following has the greatest solublity in H2O?
Which of the following has the greatest solublity in H2O?
-
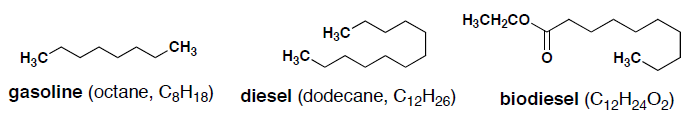
- (5 pts) Example structures of the major components of gasoline, diesel and biodiesel are shown below. Indicate which will have the highest boiling point and briefly explain your answer.
- (5 pts) Butanol is being favorably considered as an improved biofuel or fuel additive, while butylamine is not. The boiling point (and smell!) of the amine, might be part of this reason. Provide a brief explanation why butylamine has a lower boiling point than butanol.
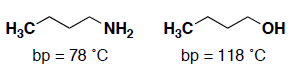
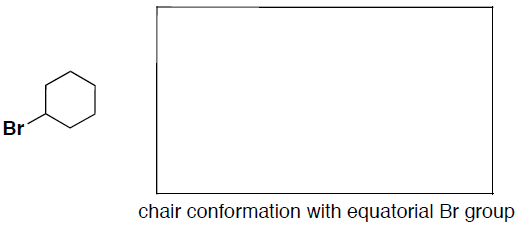
- (5 pts) Convert the flat bromo-cyclohexane structure shown below to a drawing of a chair conformation of cyclohexane with the Br group in an equatorial position. (You do not need to show hydrogens).
- (6 pts) Provide a brief explanation why a C-C single bond can freely rotate but a C=C double bond cannot. Please include any relevant orbital drawing in your explanation.
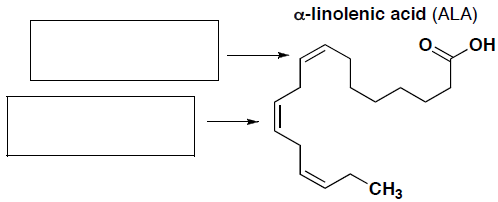
- (7 pts total) Each of the following compounds has important nutrition and health benefits. Resveratrol is an important antioxidant molecule found in the skin of grapes and a constituent of some wine. a-Linolenic acid (ALA) is an omega-3 fatty acid.
- Label whether each of the indicated alkenes in a-linolenic acid (ALA) is in the cis or trans form.
- Label whether the indicated alkene in resveratrol is the cis or trans form. Provide the name for the indicated functional group in resveratrol (in the answer box provided).
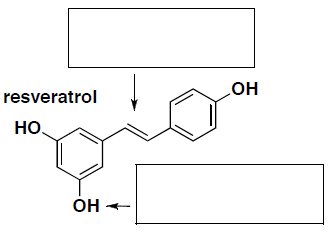
- Would you expect either of these molecules to have the same health effects if they were the opposite isomer? Briefly explain your answer.
- Extra Practice:
What intermolecular force do you think a gecko takes advantage of to stick to walls and windows?

