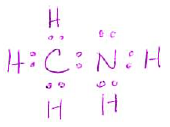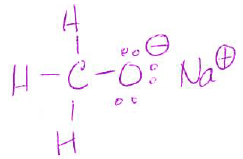3.8: Quiz 1C Key
- Page ID
- 19086
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
See comments indicating what book problems the questions relate to.
-
- Please indicate true (T) or false (F) for the following statements based on lecture materials (2 pts each):
__F__ If a carbon atom is sp-hybridized, then the bond angles will be 120°.
__T__ Oxygen is more electronegative then carbon.
__T__ The structure of acetylcholine has a Nitrogen atom with a positive charge.
- For each of the following condensed structures, indicate how many lone pairs each molecule has. (1 pt each, put your numerical answer in the box provided) (From questions 13 and 31.)
| CH3F | H2CO3 | [CH3NH3]+ | CH3OCH3 |
| 3 | 6 | 0 | 2 |
- Draw a Lewis structure for the following molecule (including any lone pairs): (4 pts)
| CH3NH2 |
|
|
or
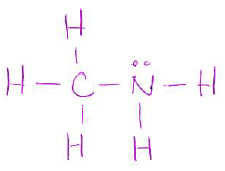
-
- (2 pts) Covalent bonds may be polar or nonpolar. What property of the atoms forming a given bond determines this?
electronegativity
- Give an example for each of the following bond types (2 pts each):
(Note: your answer does not need to be a valid Lewis structure, you can just show two atoms in a bond.)
| polar covalent bond | non-polar covalent bond |
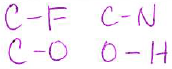 | 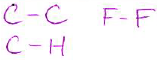 |
Many options!
- Multiple choice (2 pts each, put your letter answer in the box provided)
The compound methylamine, H3C-NH2, contains a C-N bond. In this bond, which of the following best describes the charge on the nitrogen atom? (From questions 8 and 34.)
 -1
-1- partial negative (δ-)
- uncharged (neutral)
- partial positive (δ+)
- +1
Which of the following bonding patterns is not observed for carbon?
 four pi bonds
four pi bonds- four sigma bonds
- two sigma bonds and two pi bond
- three sigma bonds and one pi bond
-
- Nicotene is an addictive substance found in tobacco. Identify the hybridization for each atom indicated. (2 pts each, put your answer in the box provided)
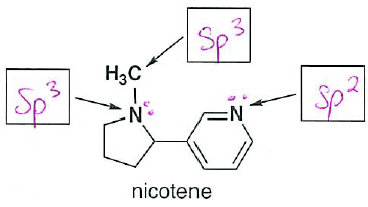
Don't forget to use lone pairs!
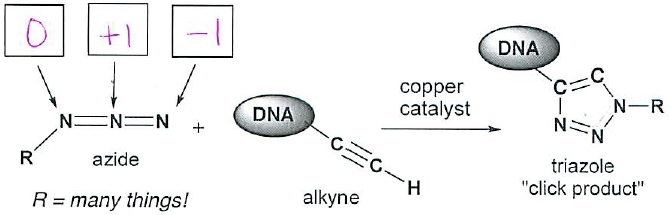
- (2 pts each) This one-step reaction between an azide and an alkyne group is called the “click reaction”. The click reaction has many examples of biological applications, such as modifying DNA for nanotechnology, discovering new drugs, or preparing new electronic sensors. Identify the formal charge on each of the nitrogen atoms in the azide molecule. (Remember to first draw lone pairs if it helps you…)
- (6 pts) Sodium methoxide (CH3ONa) is a reagent used to convert vegetable oil to biodiesel. This molecule has both ionic and covalent bonds. (From question 50.)
- Draw the Lewis or line-bond structure of CH3ONa and show all lone pair electrons and formal charges. (use box provided)
| CH3ONa |
|
|
- Which bond is ionic?
O-Na - How many covalent bonds does it have?
4 - Which bond in CH3ONa do you hypothesize is broken in the process to make biodiesel? (You have to consider reactivity, not just bond strength.)
The O-Na bond, because the electrons are shared unevenly and located on oxygen, so it will be the source of the reaction for a new bond.
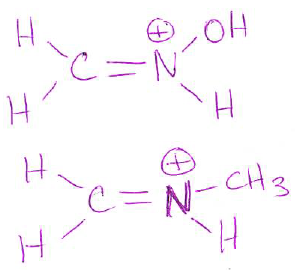
- (4 pts) Draw a structure of any molecule that has an sp2-hybridized carbon that is directly attached to an sp2-hybridized nitrogen, and the nitrogen has a positive charge. Clearly label these two atoms with the hybridization. (Note: Your molecule can have any atoms in addition to the carbon and nitrogen atoms. There are several possible answers, but make sure that your answer is a valid structure showing any hydrogens or lone pairs for full credit.)

Other groups can also be attached to N and C (instead of H).