3.30: Quiz 6A Key
- Page ID
- 19334
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
- Clearly indicate true (T) or false (F) for the following statements (2 pts each):
| __F__ | Enantiomers of a compound will have different boiling points. |
| __T__ | A chiral catalyst or enzyme can be used to synthesize a single enantiomer product. |
| __T__ | A primary carbon is less sterically hindered than a tertiary carbon, which explains why a primary alkyl halide reacts faster than a tertiary alkyl halide in an SN2 reaction. |
- (2 pts each) Multiple Choice/fill-in-the-box (choose the best answer from the options given):
- Which of the following reactions will give a racemic product?
 An achiral Rh metal complex for the hydrogenation reaction of an alkene
An achiral Rh metal complex for the hydrogenation reaction of an alkene- A reaction with the enzymes in Bakerʼs yeast
- An enzyme that only recognizes a trans alkene substrate
- A chiral Rh metal complex for the hydrogenation reaction of an alkene
- What is the chemical reason for the controversy regarding the use of methyl iodide as an agricultural fumigant?
 Methyl iodide is a racemic molecule and one enantiomer has toxic side-effects.
Methyl iodide is a racemic molecule and one enantiomer has toxic side-effects.- Methyl iodide catalyzes the hydrolysis reaction of DNA, making it toxic.
- Methyl iodide is an electrophile that will alkylate DNA, making it toxic.
- Methyl iodide makes strawberries taste bad.
- Stereoisomers with an asymmetric carbon center and non-superimposable mirror images are:
 cis/trans isomers
cis/trans isomers- enantiomers
- constitutional isomers
- end-of-the-quarter isomers
- regioisomers
- A 50:50 mixture of two enantiomers is called a ____________
 memorial day mixture
memorial day mixture- cis/trans mixture
- mirror image
- constitutional mixture
- racemic mixture
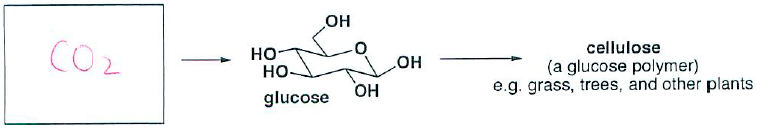
- (2 pts) What carbon building block does nature use to synthesize the following product(s)?
- Which of the following reactions will give a racemic product?
-
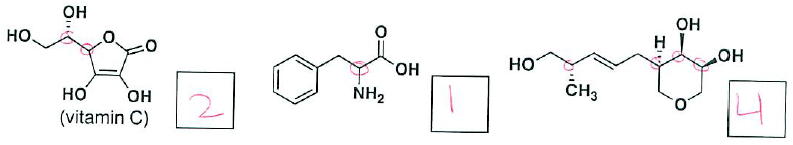
- (6 pts) How many asymmetric carbon centers does each of the following molecules have?
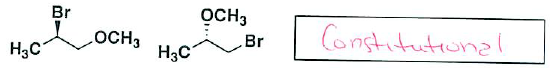
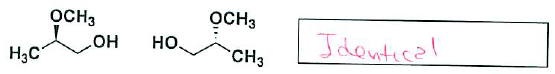
- (6 pts) Indicate for the following pairs of compounds whether they are identical, constitutional isomers, or enantiomers. (put your answer in the box provided – you may use an answer more than once, or not at all)
-
-
- (2 pts) Provide a brief definition indicating what factors make a molecule a good nucleophile.
The molecule should have high electron density (π bonds, lone pairs) that is readily available to make new bonds (delocalized e- is less available).
- (4 pts) Circle which molecule is a better nucleophile and briefly explain your answer.
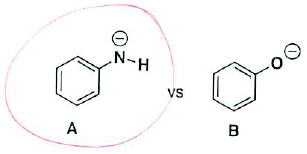
Nitrogen is less electronegative than oxygen, therefore it won't stabilize the negative charge as well, making A a better nucleophile.
-
- (5 pts) Identify the asymmetric carbon center in the molecule shown below and then draw a perspective structure for each enantiomer.

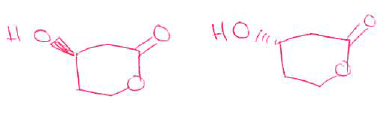
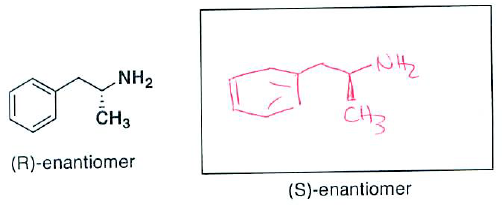
- (3 pts) The (R)-enantiomer of an amine molecule is shown below. Draw the perspective structure for the (S)-enantiomer (ie, the other enantiomer) in the box provided.
- (5 pts) Identify the asymmetric carbon center in the molecule shown below and then draw a perspective structure for each enantiomer.
- Extra Practice:
- (4 pts) Draw the racemic mixture (both enantiomers) that result upon hydrogenation of the following alkene with an achiral catalyst.
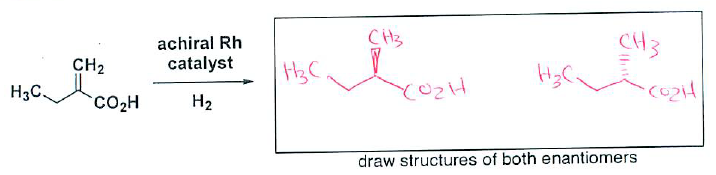
- (2 pts) How would you expect the reaction to be different if a chiral Rh catalyst is used?
You would form only one enantiomer.