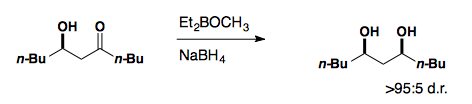Reduction of Aldehydes and Ketones
- Page ID
- 24646
Aldehydes and Ketones are reduced by most reducing agents. Sodium borohydride and lithium aluminumhydride are very common reducing agents.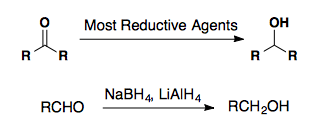
Ketones and Aldehydes can also be reduced to the respective alkanes. The Wolff-Kischner Reduction proceeds through a hydrazone intermediate under very harsh conditions. Myers developed a variation on the Wolff-Kischner reaction using a TBS-protected hydrazone which proceeds under mild conditions.
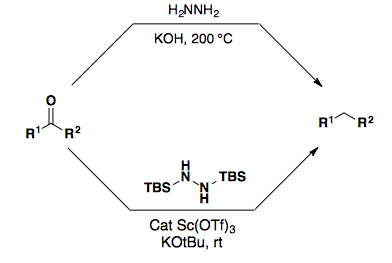
Tosyl-hydrazones can also be reduces with sodium cyanoborohydrides to yield the alkane. Additionally, a,b-unsaturated tosyl-hydrazones can be used to provide the regioisomeric alkene after a retro-ene elimination of nitrogen. This process is referred to as the alkene walk.
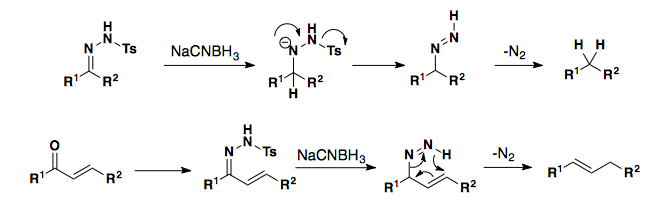
Alternatively, the carbonyl can be converted to the dithiane and reduced with Raney nickel to give the alkane product.
Chemoselective Reductions
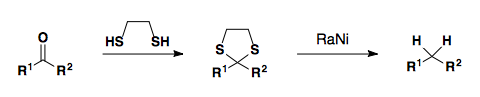
Enones present unique challenges as reducing agents can also attack the alkene giving a mixture of products. Methods to selectively reduce the ketone (Luche Reduction) and the alkene (Stryker Reduction) have been developed.
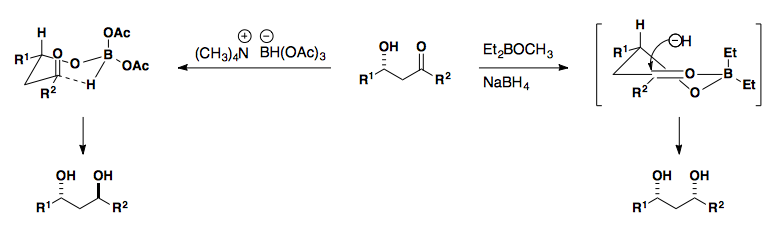
Diastereoselective Reductions
Acyclic Compounds
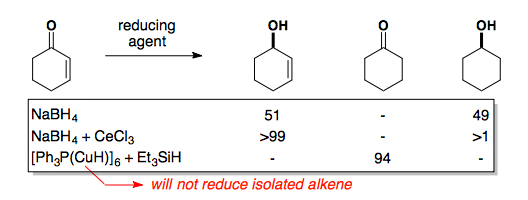
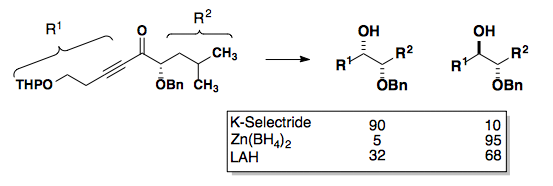
Reductions of aldehydes and ketones follow the same selectivity models as the addition of unstabilized carbon nucleophiles to these functional groups. Non-chelating reducing agents (NaBH4, LiAH4, etc.) show Felkin-Anh selectivity while reducing agents which can chelate ( Zn(BH4)2) show Cram Chelate selectivity.
Tetrahedron Lett., 1985, 26,5139-5142.
As seen in the example above, lithium will sometimes chelate.
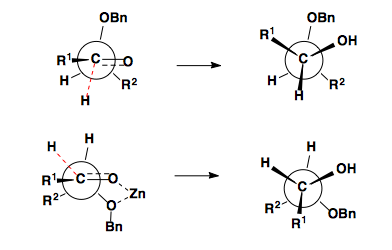
Cyclohexanones
Hydrides can approach cyclohexanones from the axial or equatorial face of the ketone.
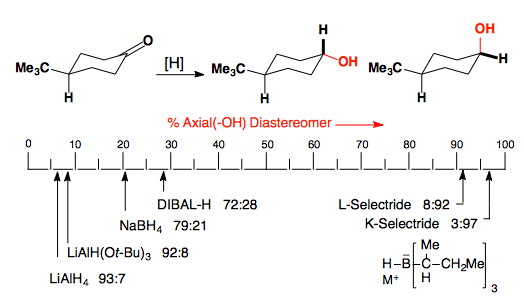
It has been obvserved that increasingly bulky hydride reagents prefer to attack from the equatorial face of the carbonyl. This is rationalized by the increased steric demand of a nucleophile approaching from the axial face of the carbonyl as it encounters the axial substituents (H in this case) at the 3 and 5 positions.
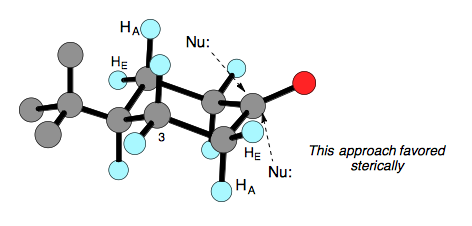
This argument would thus seem to always argue for attack from the equatorial face, but we must also take into consideration any developing torsional strain through the transition state. Attack from the axial face avoids developing eclipsing interactions between the C–O bond and the C–HE bonds at the 2 and 6 positions. Attack from the equatorial face forces the C–O bond to travel past the C–HE bonds to sit in the chair conformation. We can see this below as the dihedral angle in the starting cyclhexanone starts as a positive number and ends up as a negative number which shows that the C–O bond must have gone through an eclipsing conformation to reach its final position. Axial attack does not cause a change in sign of the dihedral angle, thus avoiding any eclipsing interactions in the transition state.
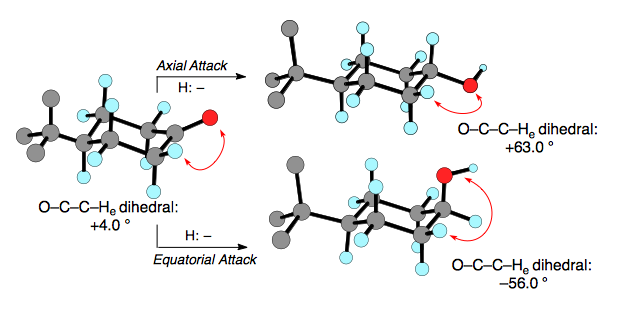
We can thus predict that small hydride reagents, such as LiAlH4, will prefer to attack from the axial face as the torsional strain in the transition state is the dominant interaction while large hydride reagents, such as H–BR4, will attack from the equatorial face as the steric interactions from the reagent's approach are now the dominant interaction.
Enantioselective Reductions
Chiral Boronates
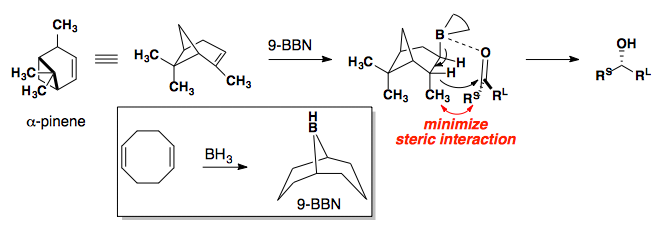
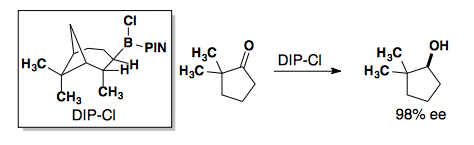
If the selectivity from these reactions is the opposite of your desired product, you can use a Mitsunobu Reaction to invert the stereocenter.
Corey-Bakshi-Shibata (CBS) Reductions
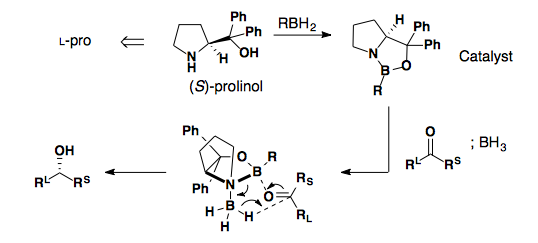
Tar-B
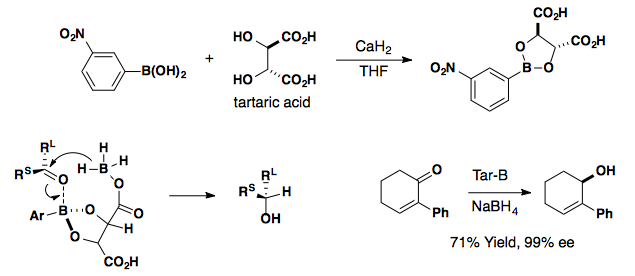


3.png?revision=1&size=bestfit&width=456&height=119)