Oxidation of Alcohols
- Page ID
- 21334
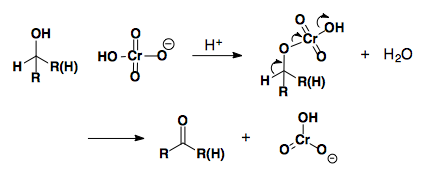
Chromium based oxidations
Typical Oxidants:
Chromium oxidants in aqueous media will oxidize primary alcohols to the corresponding carboxylic acid. The use of PCC, PDC, and Collins Reagent in methylene chloride allows for selective oxidation to the aldehyde.
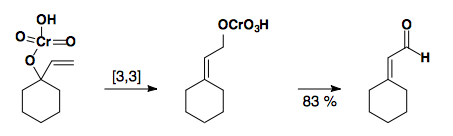
Tertiary, vinylic alcohols are known to undergo 3,3-sigmatropic rearrangements.
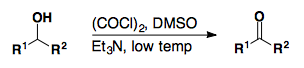
DMSO based oxidations
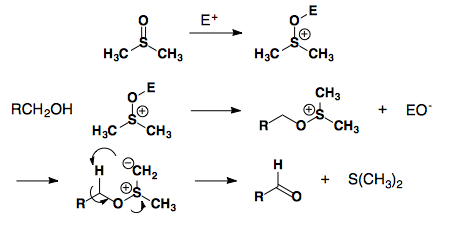
Swern Oxidation
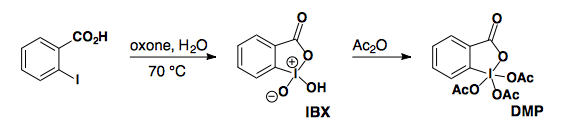
IBX/DMP
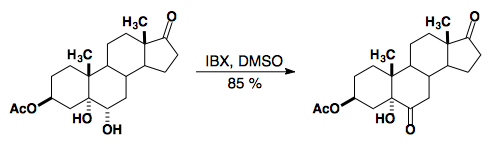
IBX can be used to oxidize 1,2-diols without C–C bond cleavage.
Selective Oxidations
1° Selective
TEMPO
Tempo can be used to oxidize primary alcohols to aldehydes.
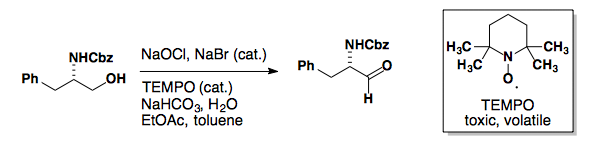
Tetrahedron Lett., 1992, 33, 5029-5033.
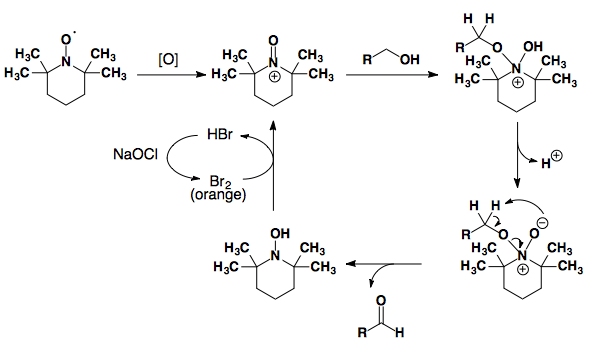
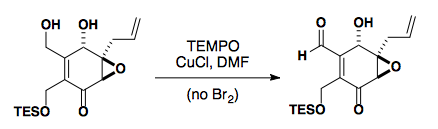
TEMPO can be used to selectively oxidize primary alcohols in the presence of secondary alcohols.
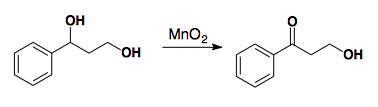
2° Selective
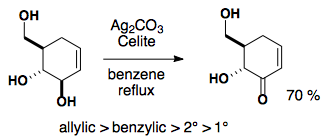
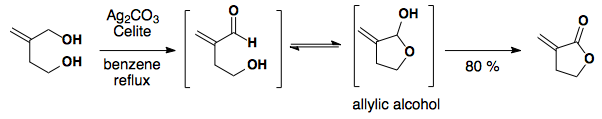
Fetizen's Reagent: Ag2CO3 on celite
Ceric ammonium Nitran (CAN)
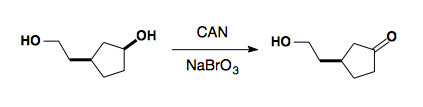
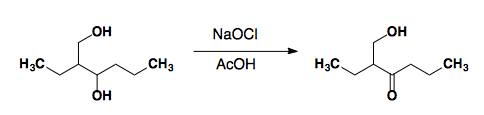
NaOCl (Bleach)
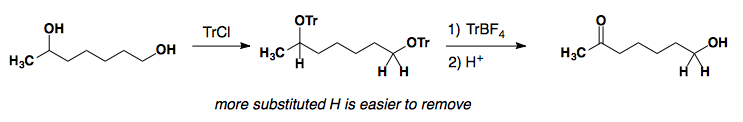
Triphenylcarbenium tetrafluoroborate (TrBF4)
Allylic/Benzylic Selective