F: Preparation of Ferrocene Fe(η⁵-C₅H₅)₂ (Experiment)
- Page ID
- 127285
Consult the following publication for background information and history: Rausch, M., Vogel, M., Rosenberg, H. Ferrocene: A Novel Organometallic Compound. J. Chem. Ed. 1957, 34, 266-272.
Recent commentary on history of ferrocene structure determination: Seeman, J. I., Cantrill, S. Wrong but Seminal. Nature Chem. 2016, 8, 193-200.
Synthesis requires the following chemicals:
4 g of KOH
12 mL of 1,2-dimethoxyethane (glyme)
1.3 g Iron(II) chloride tetrahydrate
7 mL DMSO
1.0 mL cyclopentadiene (obtained by thermal cracking of dicyclopentadiene
with storage at ca. -78ºC)
20g ice
9 mL 12 M HCl
0.70 mL Acetic anhydride
0.20 mL 85% Phosphoric acid
1.0 mL ice cold water
3 M NaOH
1mL Dichloromethane
Diethyl ether
t-Butanol
300 mg Alumina
Equipment required:
Mortar and pestle
2 Stir bars (1” and ¼”)
100 ml 3-neck RBF
3 14/20 septa
5 mL
dropping funnel (~10mL, 14/20 joint)
bubbler
250 mL beaker
25 mL beaker
600 mL beaker
Hirsch funnel
Filter paper
Adapter
Filter flask
Watch glass
2 crystallization dishes
2 Melting point tubes
10 mL round bottom flask
pH paper
column
Alumina
Stir/hot plate and heating (water bath)
Safety Notes
Perform experiment in fume hood.
DMSO, DCM, C5H5, and Et2O are all volatile and have noxious vapors. Avoid inhalation.
DMSO is easily absorbed through the skin.
KOH is hydroscopic and extremely corrosive. Handle with caution. Grind KOH in fume hood, inhalation of powder is dangerous.
HCl is a strong acid and corrosive.
Waste disposal
Use the containers provided. Needles should be disposed of in the sharps container.
Procedure
Part A: Synthesis of Ferrocene (Day 1)
Set up a 100-mL three neck (14/20) round bottom flask (RBF) with a stir bar. Attach septa on the two side necks. Use a mortar and pestle to grind 4.0 g of KOH into a powder. Do this in the fume hood to avoid breathing in powders. KOH is hygroscopic – work fast! Use a powder funnel to pour the KOH into the RBF. Add 12 mL of 1,2-dimethoxyethane through the funnel to wash the remaining KOH down. Replace the powder funnel with a dropping funnel. Seal the flask with a septum and purge it with nitrogen and use a needle as a vent. After about 10 minutes, add 1 mL of cyclopentadiene with syringe while stirring. The solution should turn pink. If the solution turns black or green, it means that small amounts of the cyclopentadiene anion has oxidized. With stirring, wait approximately 5 min then insert a venting needle into the septum to release the pressure. After the pressure has been released, remove the needle.
While the solution is stirring, pulverize 1.3 g of FeCl2·4H2O and dissolve in 6 mL DMSO in a flask. Transfer to the dropping funnel. Rinse flask with ~1mL DMSO to get quantitative transfer. While stirring, add the FeCl2·4H2O at a rate of ~20 drops per minute. After addition of FeCl2·4H2O is complete, allow reaction to stir for an additional 30 minutes.
In a 250 mL beaker, prepare 20g of ice and 9 mL of 12M HCl. Transfer a small amount to a 25 mL beaker to use to wash the reaction flask. Stop the reaction, disconnect the dropping funnel and carefully pour the reaction mixture into the HCl-ice slurry. Use the additional HCl-ice slurry in the 25 mL beaker to carefully rinse the RBF out and add this to the beaker. Stir until the mixture is dissolved and the excess potassium hydroxide is consumed. It is essential that this mixture remains near 0 °C during and after this addition. Add more ice to the solution as needed.
Set up the filtering apparatus with a Hirsch funnel, filter paper, adapter, and filter flask. Filter the beaker contents and collect the precipitate (ferrocene). Wash the ferrocene with three portions of 10mL of water. Spread the ferrocene out on a watch glass and let it dry until the next lab.
Part B: Purification of Ferrocene (Day 2)
Ferrocene is a crystalline, diamagnetic material that is extremely stable to air, moisture and light. It is moderately to extremely soluble in practically all-nonpolar or weakly polar solvents. It may be purified by sublimation.
Weigh and take a melting point of the dried crude ferrocene. [NOTE: All melting points must be taken in melting point tubes sealed off with parafilm. Ferrocene sublimes below its melting point and would be lost from an unsealed tube.]
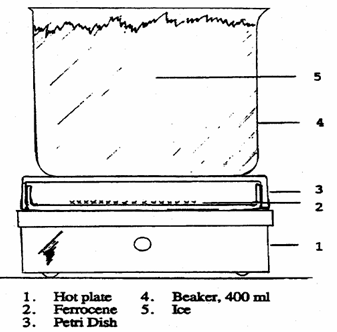
Sublimation may be conducted in a 100 x 15 mm culture dish as shown in the figure below. Transfer ferrocene to the "bottom" of the culture dish to cover the center of the dish to a thickness of about 5 mm. Cover with the larger half of the culture dish and place it on a variable temperature hot plate. Slowly raise the temperature until the ferrocene sublimes to the upper half of the dish. The sublimation will proceed slowly. Cooling the top culture dish by placing a beaker filled with ice water on top of it will facilitate the sublimation (WARNING: Slide the beaker off the top of the culture dish. Lifting it may lift the upper culture dish off and cause it to fall or disturb the sublimed ferrocene resulting in a loss of ferrocene.) Remove the dish from the heat, allow it to cool and recover the sublimed ferrocene. This procedure may be repeated several times until all the ferrocene is purified. Do not heat over 100 °C. To prevent sublimated ferrocene fall of when trying to recover them, when there is a layer of ferrocene on the top of the culture dish, students can take it off an dput another upper culture dish on top, Repeat this until no orange product on the bottom.
Determine the melting point of each batch of ferrocene sublimed. Place the final product in a weighed vial, determine the yield and report this along with the melting point. Calculate and report your actual percent yield. The melting point should equal or exceed 171 °C (lit., 173- 174 °C).
Part C: Synthesizing Acetylferrocene & Chromatography
You will acetylate ferrocene using phosphoric acid and acetic anhydride and determine the number of products with thin layer chromatography. If we don’t have enough ferrocene, scale down every reagent based on the ferrocene that will be used in this part.
Prepare a 65 °C water bath and preheat a 10 mL round bottom flask with a stir bar. Add 1.0 mmol of ferrocene, 0.7 mL of acetic anhydride and 0.2 mL of 85% phosphoric acid. If you do not add the reactants in this order, you will successfully decompose the ferrocene. Close the round bottom with a septum and insert a venting needle. Warm the solution with stirring until the ferrocene dissolves, and then heat the solution for 30 minutes. Thoroughly cool the round bottom in an ice bath and then carefully add 0.5 mL of ice water to the mixture with stirring. Add drop wise 3 M aqueous sodium hydroxide solution until the solution pH is neutral (somewhere between 20-30 drops). Collect the product in a filter funnel and wash the solids with water. Press the product with filter paper to get it as dry as possible. The crystals air dry. Take a yield and melting point of the crude product.
Part D: Procedure for TLC & Column Chromatography
You will determine the distribution of products in your acetylation reaction using thin-layer chromatography.
Prepare solutions of your sublimed ferrocene and dried acetylation product by placing a few (5 to 10) drops of methylene chloride, CH2Cl2, in two small vials. Add a small amount (one spatula-tip full) of ferrocene to one vial and acetylation product to the other vial. The solution should be concentrated enough so that a dark spot is clearly visible on the TLC plate after application. If you are unable to see spots on the TLC plate, add more sample to the solutions and try again (You can use the same TLC plate, just apply the more concentrated solution directly on top of where you applied the first sample.)
The goal of this portion of the experiment is to determine the best hexane to ethyl acetate ratio that most effectively separates the various ferrocene products. The principle applied here is the separation of products as a function of solvent polarity.
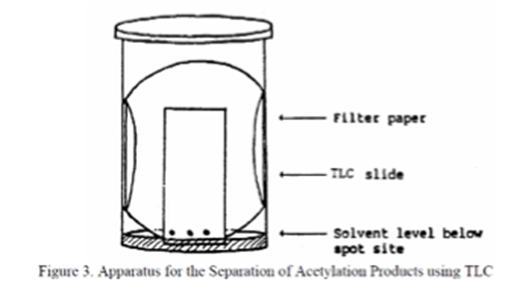
Begin by testing product separation with pure solvent (hexane or ethyl acetate). Once you have obtained those results, decide on whole number ratios of solvent mixtures and test those in the same way (i.e. 1:4 hexane to ethyl acetate). TLC plates may be obtained from the dispensary and you will use micro-capillary tubes as applicators. Put a piece of filter paper in a small jar with lid as shown in the Figure and carefully pour in the solvent. Be certain that the solvent level is below the point where you spot the TLC plate. Draw a line to mark the starting solvent on the TLC plate, then use the capillary to spot the TLC plate slightly above the solvent line. Carefully place the spotted TLC plate into the solvent jar and place a lid on top. Let the plate develop in the solvent until the product spots are within 1 cm from the top of the plate. If you leave the plate in the solvent for too long, the spots will collect at the top of the plate and you will have no useful observations. Sketch each chromatogram results in your lab notebook along with a note of which solvent ratio you used for that plate. Once you obtain a TLC plate of good separation (no smearing of spots, no spot overlap etc.), calculate the ratio to front (Rf) value for each spot.
At this point you would then perform column chromatography with your TLC determined solvent ratio in order to collect your isolated products and determine their purities and yields. Unfortunately, time constraints in 124L do not leave enough time for you to do this. But please make note in your lab report, how you would perform such an experiment and what you would do to determine purity and yield of your separated products.