11: Metal-Metal Quadruple Bonds (Experiment)
- Page ID
- 127271
Synthesis of Chromium (II) Acetate
Safety Notes
This procedure requires the use of concentrated (12 M!) HCl(aq) which is corrosive, causes burns and releases HCl gas, which is also toxic and corrosive and causes burns. Care must be taken that this material is kept in the hood at all times.
The addition of HCl to Zn metal produces H2 gas which is extremely flammable, odorless, and colorless. The gas produced by the reaction must be vented into the hood, and there are to be absolutely no Bunsen burners or sparks of any kind during this experiment.
Chromium (III) is toxic and an irritant, so this material is not to be allowed in the sinks or the drains. The chromium (II) produced by this experiment is a strong reducing agent, and so must be treated with caution. If it will reduce oxygen, it will reduce you, too. Do not pour this waste down the drain, there will be a specially labeled waste container instead.
As always, goggles MUST be worn in the lab at all times. You only have two eyes and they are not replaceable. Also, because of the toxicity and reactivity of the chemicals used in this experiment, it is strongly advised to wear gloves whenever handling any chemical and good chemical hygiene (common sense) is expected at all times.
Handling:
It is important to remember that chromium (II) is very sensitive to oxygen, so the reaction must remain under inert gas at all times. This reaction also produces a lot of H2 gas, and you must be careful to make sure that the gas has somewhere to go. Otherwise your glassware may decide to fly apart, and aside from ruining your experiment, it could create a serious accident. Even with venting, enough pressure may build inside the flask to make connections pop loose, so be sure to secure any attachments to your flasks with rubber bands.
Because of the sensitivity of chromium (II), all solvents and reagents (15mL DI water, 32 mL conc. HCl, 25mL saturated NaOAc soluiton) must be thoroughly degassed by bubbling N2 through them for at least 30 min. Do not use tap water, or dissolved O2 in the liquid will destroy your product.
Procedure:
We will be using a Schlenk line in this experiment, so you need to make sure that the line is ready before you begin working. The line itself should resemble Figure 3, but you will want to have your TA show you what needs to be done if you don’t already know. You should have N2 flowing through the gas side of the Schlenk and the vacuum pump should also be ready before you begin.
Find a 250 mL Schlenk flask and clamp it into position in an empty pan on top of a magnetic stir plate. Add to it a magnetic stir bar, 22.6 g Zn metal, 13.7 g CrCl3·6H2O, and 15 mL of degassed, deionized water. Connect a 50 mL pressure equalizing addition funnel, and close the free neck with a glass stopper. Don’t forget to grease all the ground glass connections! It should look like the setup in figure 1. Connect the apparatus to the Schlenk line with a thick Tygon hose, then evacuate the apparatus and refill it with N2. Repeat this step several times, or some O2 will remain in the flask. With N2 flowing through the flask from the sidearm, pour 32 mL degassed, concentrated HCl(aq) into the addition funnel.
When you have finished setting up the reaction, cool the flask with a bath of ice and water, then begin stirring. The CrCl3·6H2O should dissolve to give a dark green solution. Once the mixture has had time to cool and the CrCl3·6H2O has dissolved, check to make sure that the flask is open to the N2 line (very important!). If it is, begin dripping the HCl into the solution. You should see a very vigorous and exothermic evolution of H2 gas, and after a few minutes, the reaction will start to turn very bright blue.
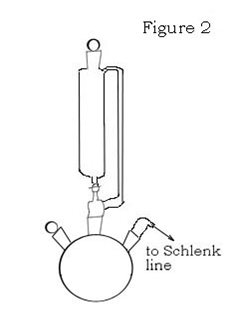
While the reaction is running, you will need to setup another apparatus. This one will be a 3-neck round bottom flask (1L), a second addition funnel, another magnetic stir bar, 2 glass stoppers and gas inlet. Connect the addition funnel to the middle neck of the flask, the gas inlet to one side neck and glass stoppers in the open joints. The apparatus should look like figure 2. Connect the gas inlet to the Schlenk line with a length of Tygon hose. Evacuate and refill the flask with N2 several times, then close it off from the Schlenk line.
After all of the HCl has been added to the reaction flask, you will notice that the evolution of gas begins to slow down. When the evolution is complete or close to completion, replace the addition funnel with a rubber septum (with N2 flowing through the flask), and replace the glass stopper on the side of the 3 neck flask with a rubber stopper as well (don’t forget the N2!!). You are now ready to transfer the solution to the empty flask. This will be done with a cannula, which is a very long, double ended needle. Poke the cannula through the rubber septum of the reaction flask and allow N2 to flow through it for a few seconds. You will then poke the other end of the needle through the rubber septum on the empty flask. Lower the end of the needle into the reaction mixture, and clamp the whole flask higher than the receiver. Use a lab jack to support the raised flask, and make sure the receiving flask is resting on something secure. To get the solution to siphon into the empty flask, quickly turn the Schlenk key of the empty flask past the vacuum position, but beware that dissolved HCl and H2 will try to bubble out from the solution! If you’re not sure about this step, find your TA.
Once all of the CrCl2 solution has been transferred to the receiving flask, it should be open to the N2 line if it’s not already and you are done with the reaction flask. Cannulation won’t transfer everything, so some excess Zn and a little bit of solution will remain behind – this is OK. Dispose of the solids and liquid properly, and make sure that all of the equipment is clean for the next group. Your TA will know where to dispose of the waste. While you’re at it, you may want to observe how quickly your CrCl2 changes back to green! This is an indication of how careful you need to be with your experiment. If you observe this color change during the next few steps, you probably let air into your flask and you should talk to your TA. Be sure to clean the cannula and flask with water right away (regular distilled water is fine this time) because you will reuse it in the following step.
With N2 flowing through the flask, open the glass stopper on the addition funnel and pour in 25 mL of degassed, saturated sodium acetate solution. Start the magnetic bar stirring and slowly add the sodium acetate solution. You will begin to see a dark precipitate which is Cr(O2CCH3)2. We now need to isolate the precipitate, and this will be accomplished by using a Schlenk filter, shown in Figure 4.
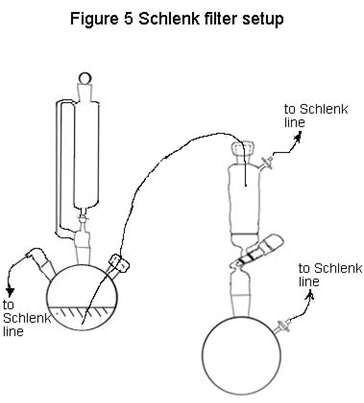
Evacuate and fill the both sides of the Schlenk filter several times, then with N2 flowing through the flask, replace the glass stopper with a rubber septum. While the reaction vessel is open to the N2 line, poke the clean cannula through the rubber septum. Allow N2 to flow through for a few seconds, and then insert the free end of the needle through the septum of the filter. Your setup should resemble Figure 5. Cannulate the solid and the liquid into the top of the filter, then have your TA show you how to use the Schlenk filter. Remove as much of the liquid as you can with the vacuum pump, then collect the solids. Measure IR spectrum of the product with the solid.
In your report, you should include the IR spectrum for the product, as well as the yield. Also, be sure to include an analysis of how the two chromium atoms are interacting using literature references that are not included in Experiment 11 in your lab manual.