Cytochrome P450
- Page ID
- 2486
Cytochrome P450 is an enzyme super family that often catalyzes physiologic processes. It consists of a minimum of two protein components: Cytochrome p450 and a flavoprotein called NADPH: 450 oxidoreductase3. Cytochrome P450 enzymes are heme-containing proteins that often hydrolyze many organic molecules in animals1. It is well know (known, spelling error fixed by Huy Phan at 11:50)_ that heme contains iron as a source/sink of electrons for reduction/oxidation reactions. There are many explainable dimensional characteristics of Cytochrome P450 showing how the metal core interacts, influences, and participates in chemical processes in an organism.
Heme Protein in Cytochrome P450
In the 1950’S Hayaishi developed and demonstrated how oxygen was involved with the substrate. The iron-containing porphyrin molecule contributed to activation and transfer of oxygen to the substrate. Because CYP450 (Cytochrome P450) is a hem-containing metalloenzyme, the iron is tethered to a thiolate side chain of a cysteine within a protein5. Another characteristic of the heme is the heavily conjugated ring system that allows for electron stability, which leads to catalytic intermolecular reactions.
Cysteine Thiolate
A critical step in CYP450’s reaction is when a O-O-Fe cleaves the O-O with the aid of the thiolate that is on the side chain of the cysteine. Dawson postulated that the thiolate is available as a good donor, thus exerting a push effect that would enable it to cleave the O-O bond. The cleavage of this bond puts Cytochrome P450 into a species that is believed to a Fe-O compound complex that limits the cycle and enables it to progress6.
*cysteine ball and stick via wikipedia
Fe Symmetry in Cytochrome P450 Heme
With the iron at the center of the heme molecule in CYP450, the symmetry as it relates to just the metal is tetragonal7. When the heme has the iron attached, the heme has 4 covalent bonds to nitrogen counterparts with P electrons contributing to its stability. This porphyrin molecular conjugation is a modified group of four pyrrole subunits attached by methane bridges to the alpha carbon of the pyrrole. As shown in the screen shot below of human Cytochrome P450 3A4 (via Ligand Explorer and PDB.org) you can see the cys442 has a metal interaction with the iron at a distance of 2.35Å. With this bond distance in Cytochrome P450 3A4 the crystal structure show the ability for the thiolate (cys442) to interact with the heme. The symmetry of Cytochrome P450 is difficult to characterize due to the diversity of the metalloenzyme family. Most heme proteins, no exception to CYP450, experience some planarity deviation1.
Raman and IR Spectroscopy
DFV Lewis, in 1996, published Cytochromes p450: structure, function and mechanism and showed that Raman Spectroscopy data gave the following results for Fe(II) and Fe(III):
|
| Frequency Range(cm-1)
| ||
| Mode
| Symmetry
| Fe(III)
| Fe(II)
|
| V10
| B1g
| 1623-1637
| 1600-1612
|
| V37
| Eu
| 1580-1601
| 1584-1586
|
| V19
| A2g
| 1583
| -----------------------
|
| V2
| A1g
| 1565-1584
| 1556-1564
|
| V11
| B1g
| 1549-1564
| 1532-1534
|
| V38
| Eu
| 1548-1550
| 1521
|
| V3
| A1g
| 1485-1502
| 1462-1466
|
| V28
| B2g
| 1464-1465
| 1445
|
| V4
| A1g
| 1370-1373
| 1341-1344
|
*RR spectroscopy data of Fe II and III states9.
IR and Raman are both conducted, but Raman data is more used for CYP450 because it can be used to quantify the different transition states of the metalloenzyme more effectively
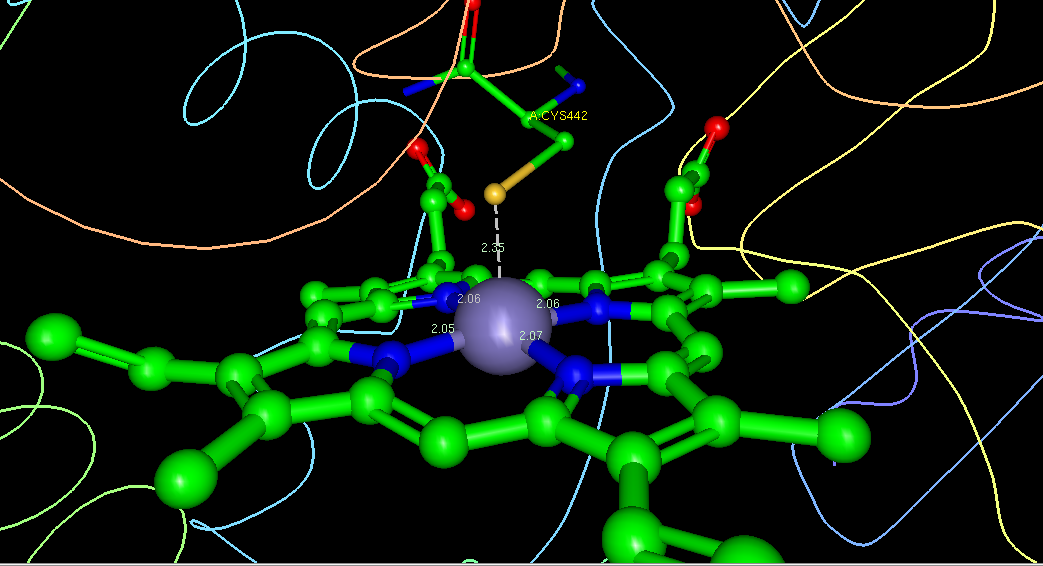
*mol. wt. 616.48 C34H32FeN4O4, Screen Shot of Heme interaction with the protein ligand thiolate and Fe. Courtesy of PDB.org and Ligand Explorer(CYP450-3A4)
References
- Poulos, T, Finzel, B, & Howard, A. (1986). High-resolution crystal structure of cytochrome p450cam. Journal of Molecular Biology, 195, 687-700.
- Prahl, Scott. (1998). Structure of heme. Retrieved from http://omlc.ogi.edu/spectra/hemoglobin/hemestruct/index.html
- Foye, William, Lemke, Thomas, Williams, David, & Reviews, Cram101. (2009). Outlines & highlights for foyes principles of medicinal chemistry by lemke, thomas l / williams, david a, isbn. Philadelphia, PA: Academic Internet Pub Inc.
- Harris, G. (1968). Spin-mixing and the different spin states of ferric ion in tetragonal symmetry . Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta), 10, 155-180.
- Ortiz de Montellano, P. (2005). Cytochrome p450: structure, mechanism, and biochemistry. New York, NY: Springer-Verlag New York, LLC.
- Ogliaro, F, de Visser, S, & Shaik, S. (2002). The ‘push’ effect of the thiolate ligand in cytochrome p450: a theoretical gauging . Journal of Inorganic Biochemistry, 9, 554-567.
- Winkler, J, Wittung-Stafshede, P, Leckner, J, Malmstrom, B, & Gray, H. (1997). Effect of folding on metalloprotein active sites. Proceedings of the National Academy of Sciences, 94, 4246-4249.
- Smith, S.J., Munro, A.W., & Smith, W.E. (2003). Resonance raman scattering of cytochrome p450 bm3 and effect of imidazole inhibitors. Bipolymers, 70(4), 620-627.
- Lewis, DFV. (1996). Cytochromes p450: structure, function and mechanism. London and Bristol, PA: Taylor and Francis.

