Midterm 2
- Page ID
- 50496
Problem 1 (2 points each = 20 points total)
MARK the following statements as TRUE or FALSE.
 The Pauli Exclusion Principle states that two or more electrons in a single atom must have the same four quantum numbers.
The Pauli Exclusion Principle states that two or more electrons in a single atom must have the same four quantum numbers.
 At absolute zero, all intermolecular and intramolecular motion has ceased.
At absolute zero, all intermolecular and intramolecular motion has ceased.
 The square of the wavefunction is measure of the energy of the wavefunction.
The square of the wavefunction is measure of the energy of the wavefunction.
 The Bohr Principle states that an atom is wavelike only when it has enough mass to exceed the Bohr radius.
The Bohr Principle states that an atom is wavelike only when it has enough mass to exceed the Bohr radius.
 Amino acids are never part of the active site of an enzyme?
Amino acids are never part of the active site of an enzyme?
 The number of nodes in a wavefunction is typically correlated to the energy of that wavefunction.
The number of nodes in a wavefunction is typically correlated to the energy of that wavefunction.
 According to Einstein’s Law of photoelectron effect in a primary photoionization process of a metal, each electron is generated by the absorption of a single photon.
According to Einstein’s Law of photoelectron effect in a primary photoionization process of a metal, each electron is generated by the absorption of a single photon.
 Enzymes can make a reaction go faster by reducing DG for the reaction.
Enzymes can make a reaction go faster by reducing DG for the reaction.
 Hydrogen-like wavefunctions are derived from solving the Schrodinger equation for a charged nucleus with a single moving electron.
Hydrogen-like wavefunctions are derived from solving the Schrodinger equation for a charged nucleus with a single moving electron.
 The particle in the box model predicts that the particle has a small probability to be found outside the box as long as the potential walls surrounding the box are of finite height.
The particle in the box model predicts that the particle has a small probability to be found outside the box as long as the potential walls surrounding the box are of finite height.
Problem 2 (10+10=20 points)
2A) What is the energy of a particle with mass of 2.3 x 10-27 kg that is confined in a one-dimensional box of 50 Å length? Assume that the particle is in the third energy level.
2B) What is the wavelength of a photon released during a transition from level three to level two in above system?
Problem 3 (10 + 5 points = 15 total)
3a) The speed of a hydrogen atom is 350 km s-1. If the uncertainty in its momentum is 0.01%, what is the uncertainty in its location? If a hydrogen atom has a diameter of 1.4 Å, how large is this uncertainty compared to its size (i.e., in percent?)
Problem 5 (10 points each = 20 points)
B) A reaction that consume CO2 follows Michaelis-menten enzyme kinetics with Km=0.001 M, and the initial concentration of substrate=0.840 M. After 2 seconds, 5% of the substrate has been converted to product[DL1] . How much CO2 will be converted after 10 and 60 seconds?
3B) When 2 x 10-10 mol is added to a 1-L solution containing its substrate at a concentration one hundred times the Km, it catalyzes the conversion of 75 µmol of substrate into product in three minutes. What is the enzyme’s turnover number (turnover rate)?
Problem 6 (2 + 5 = 8 points total)
Answer the following questions using the diagrams shown for enzymatic catalyzed reactions.
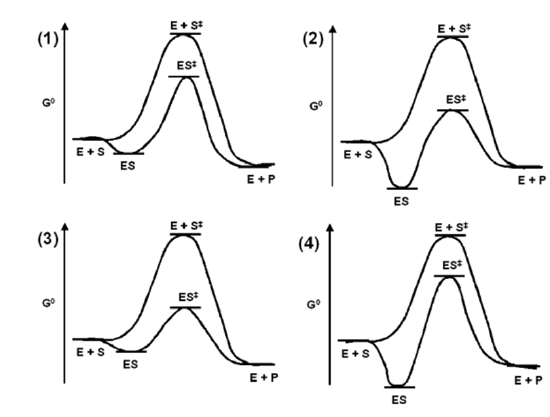
- Which enzyme is providing the least acceleration in relationship to the non-catalyzed reaction?
- Which enzyme is providing the greatest acceleration in relationship to the non-catalyzed reaction?
- When comparing enzymes 1 and 4, which will be faster?
- When comparing enzymes 2 and 3, which will be slower?
Problem 7 ( 6 + 3 + 3 = 12 points)
7A) How many electrons in a hydrogen atom can occupy the wavefunctions with the following values of l:
- l =0
- l =3
- l =5
7B) What is the probability of finding a particle outside a box (choose either: 0, 1 or in-between)
- with finite potential walls and why?
- with infinite potential walls and why?
7C) What is the probability of finding a particle exactly in the middle of a box (with infinite potential walls) when excited to the n=1 state?
Problem 8: 10 points
The diffraction phenomenon can be observed whenever the wavelength is comparable in magnitude to the size of a slit opening. How fast must your instructor, weighing 92 Kg, run to diffract through a door 1 m wide?

