3.19: Quiz 3C
- Page ID
- 19275
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
- Clearly indicate true (T) or false (F) for the following statements (2 pts each):
| _____ | A chair conformer with substituents in the equatorial position is less stable. |
| _____ | The stronger the acid, the more stable the conjugate base. |
| _____ | Cyclopropane has more ring strain than cyclohexane, which is why molecules containing cyclopropane are often used for rocket fuel or explosives. |
- Give the best answer for the following multiple choice questions: (2 pts each)
Indicate which of the following molecules has a conjugated pi system.
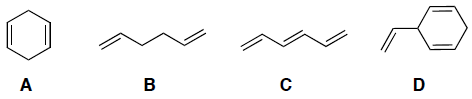

Rank the following in order of deceasing λmax (i.e. decrease in nm):

- A>B>C>D
- D>A>B>C
- A>D>C>B
- B>C>D>A
- D>B>A>C
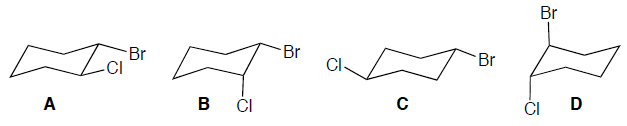
Which of the following represents a cis isomer of cyclohexane?

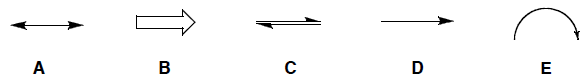
Which arrow is used to represent the movement or delocalization of electrons?

- (8 pts) Benzene and cyclohexane are both important 6-membered ring carbon structure that are predominant in pharmaceutical molecules and other molecules of biological interest. Draw the structure for each and then provide 3 chemical facts (i.e. orbitals, hybridization, conformation, etc) for each molecule that indicates the important differences in their structures.
| benzene | cyclohexane |
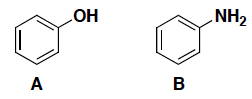
- (6 pts) Which compound (A or B) is a stronger acid? In each case, circle your answer and provide a very brief explanation for your answer. (In this case, a simple correct phrase is sufficient for your answer…)
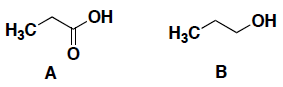
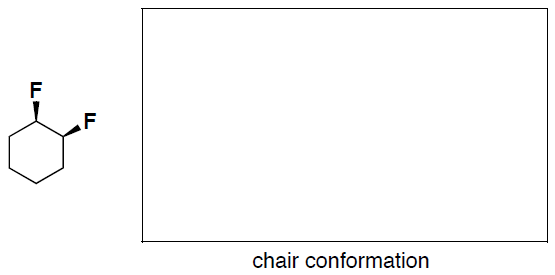
- (6 pts) Convert the flat difluorocyclohexane structure shown below to a drawing of a chair conformer. Clearly label if the fluoro groups are axial or equitorial in your chair structure.
- Delocalized electrons and resonance (10 pts total)
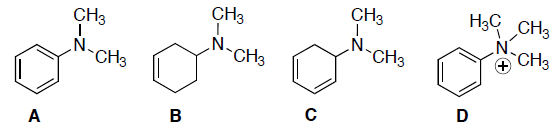
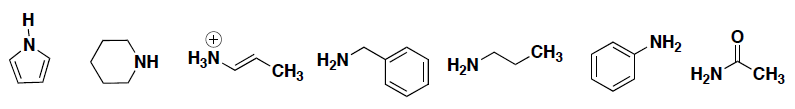
- Circle any of the following compounds that have a nitrogen atom with delocalized electrons.
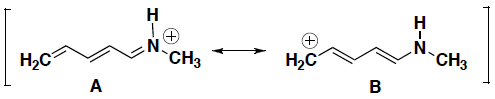
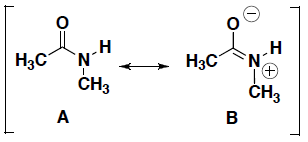
- For each pair of resonance structures shown below (A and B), circle which resonance contributor makes a greater contribution to the resonance hybrid. Briefly explain your answer.