Worksheet 5A: Quantization
- Page ID
- 36950
Q1
Three related questions.
- Why are ultraviolet, x‑rays, and gamma rays always regarded as harmful while infrared, microwaves, and radio waves are generally regarded as benign (by sensible people, anyway).
- In what sense can visible light be thought of as "just right" as an energy source for life (and other forms of chemistry) on earth?
- Under what circumstances could exposure to the generally benign electromagnetic waves listed in part a. be considered harmful?
Q2
Which photons have more energy, those in blue light or those in red light?
Q3
A microwave oven emits 700 W (Joules /sec) of radiation at a frequency of 2.45 GHz. How many photons does it generate per second?
Q4
Complete the table.
| Energy per photon (J) | Energy per mole of photons (J/mol) | Wavelength of light (nm) | Frequency of light (1/s) | Type of Radiation |
| 300 | Ultraviolet | |||
| \(1.0 | ||||
| \(1.0 | ||||
| \(4.0 |
Q5
The color temperature of a light source is the temperature of an ideal black-body radiator that radiates light of comparable hue to that of the light source. The following table relating the temperature of the surface of stars to the color description of their emitted light, which is well described as a black-body radiator. Estimate the temperature of the surface of our star, the sun.
| Effective temperature | Color description |
|---|---|
| ≥ 30,000 K | blue |
| 10,000–30,000 K | blue white |
| 7,500–10,000 K | white |
| 6,000–7,500 K | yellow white |
| 5,200–6,000 K | yellow |
| 3,700–5,200 K | orange |
| 2,400–3,700 K | red |
| 1,300–2,400 K | red brown |
| 500–1,300 K | brown |
| ≤ 500 K | dark brown |
Q6
What property (or properties) of waves involves in the photoelectric effect, demonstrates the quantized nature of light (i.e., the existence of photons)?
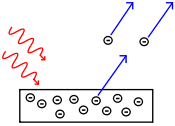
Q7
Find the de Broglie wavelength for an electron moving at the speed of 5.0 x 106 m/s (mass of an electron = 9.1 x 10-31 kg).
Q8
Complete the following table
| Object | Mass (Kg) | Velocity | Momentum | de Broglie Wavelength (m) | \(\Delta{x}\) (m) | \(\Delta{v}\) (m/s) |
| Baseball | 0.15 | 10 m/s | 1.5 | \(1 \times 10^{-10}\) | \(5.27 \times 10^{-25}\) | |
| Virus | \(2 \times 10^{-17}\) | 10 m/s | \(1 \times 10^{-10}\) | |||
| Electron | \(9.11 \times 10^{-31}\) | 10 m/s | \(1 \times 10^{-10}\) |
Q9
Calculate the minimum uncertainty in the speed of an electron that can move along the carbon skeleton of a 5.0 nm long polymer.

