Homework #5
- Page ID
- 36914
Show your work for calculations.
Q1
What is the frequency (in s-1) associated with electromagnetic radiation of 200 pm wavelength? Identify the type of radiation based on below figure.
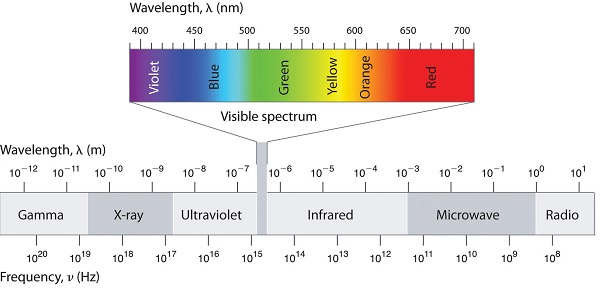
Q2
Which photons have higher energy?
- Photons with \(100\; nm\) wavelength
- Photons with \( 10\; \mu m\) wavelength
Q3
An electron of \(He^+\) ion transitions from \(n=2\) to \(n=8\) state when it absorbs light. What is the wavelength of light (in nm)?
Q4
Calculate the third ionization energy of lithium in kJ/mol.
Q5
Find the de Broglie wavelength (in pm) for an electron moving at the speed of \(6.0 \times 10^6\; m/s\) given the mass of an electron is \(9.1 \times 10^{-31}\; kg\).
Q6
For the electron discussed in Q5, if its speed can be measured with the precision (uncertainty) of 0.50 %, what is the uncertainty in the position of the electron (in nm)?
Q7
If the proton is traveling at the same speed as the electron in Q5, is the de Broglie wavelength of the proton longer or shorter than the value calculated in Q5? Explain why.
Q8
A particle is confined to a one-dimensional box with a length of 4.00 nm (\(0.00\; {\rm nm}\le x \le 4.00\; {\rm nm}\)). If the particle happens to be at the \(n=5\) state, at what points does the particle has zero probability of being found? Draw the graph of \(\psi^2(x)\) versus \(x\) and explain the result.

