4.1: Physical Principles
- Page ID
- 367409
Photoelectron spectroscopy utilizes photo-ionization and analysis of the kinetic energy distribution of the emitted photoelectrons to study the composition and electronic state of the surface region of a sample. Traditionally, when the technique has been used for surface studies it has been subdivided according to the source of exciting radiation into :
- X-ray Photoelectron Spectroscopy (XPS) - using soft x-rays (with a photon energy of 200-2000 eV) to examine core-levels.
- Ultraviolet Photoelectron Spectroscopy (UPS) - using vacuum UV radiation (with a photon energy of 10-45 eV) to examine valence levels.
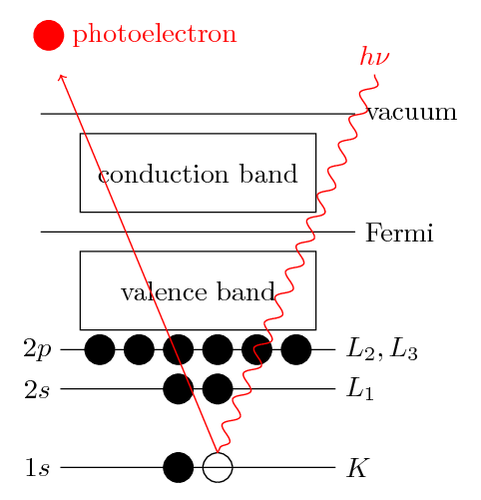
The development of synchrotron radiation sources has enabled high resolution studies to be carried out with radiation spanning a much wider and more complete energy range ( 5 - 5000+ eV ) but such work remains a small minority of all photoelectron studies due to the expense, complexity and limited availability of such sources.
Physical Principles
Photoelectron spectroscopy is based upon a single photon in/electron out process and from many viewpoints. The energy of a photon of all types of electromagnetic radiation is given by the Einstein relation :
\[E=h \nu \nonumber \]
where \(h\) is Planck constant (\(6.62 \times 10^{-34}\, J \cdot s\)) and \(\nu\) is the frequency (Hz) of the radiation.
Photoelectron spectroscopy uses monochromatic sources of radiation (i.e. photons of fixed energy). In XPS, the photon is absorbed by an atom in a molecule or solid, leading to ionization and the emission of a core (inner shell) electron. By contrast, in UPS the photon interacts with valence levels of the molecule or solid, leading to ionization by removal of one of these valence electrons.
The kinetic energy distribution of the emitted photoelectrons (i.e. the number of emitted photoelectrons as a function of their kinetic energy) can be measured using any appropriate electron energy analyzer and a photoelectron spectrum can thus be recorded. The process of photoionization can be considered in several ways : one way is to look at the overall process as follows:
\[\ce{A + h\nu → A^{+} + e^{-}} \nonumber \]
Conservation of energy then requires that :
\[\ce{E(A)} + h\nu = \ce{E(A^{+} ) + E(e^{-})} \label{eq2} \]
Since the electron's energy is present solely as kinetic energy (\(\ce{KE}\)) this can be rearranged to give the following expression for the KE of the photoelectron:
\[\ce{KE = h\nu - [E(A^{+} ) - E(A)]} \nonumber \]
The final term in brackets, representing the difference in energy between the ionized and neutral atoms, is generally called the binding energy (\(\ce{BE}\)) of the electron - this then leads to the following commonly quoted equation :
\[\ce{KE = h\nu - BE} \nonumber \]
An alternative approach is to consider a one-electron model along the lines of the following pictorial representation ; this model of the process has the benefit of simplicity but it can be rather misleading.
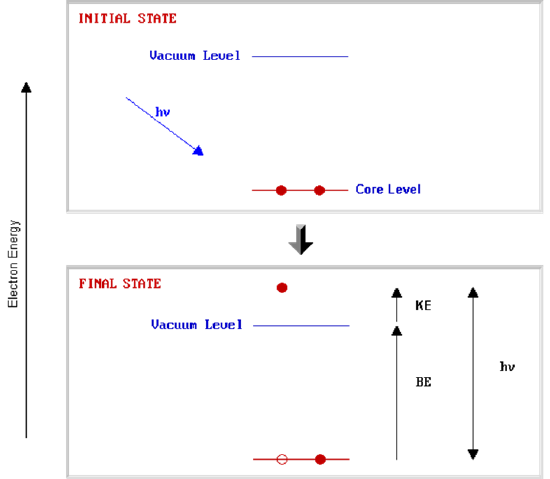
The BE is now taken to be a direct measure of the energy required to just remove the electron concerned from its initial level to the vacuum level and the \(\ce{KE}\) of the photoelectron is again given by :
\[\ce{KE = h\nu - BE} \nonumber \]
The binding energies (BE) of energy levels in solids are conventionally measured with respect to the Fermi-level of the solid, rather than the vacuum level. This involves a small correction to the equation given above in order to account for the work function (φ) of the solid.
At the most fundamental level ionization energies are well-defined thermodynamic quantities related to the heats of protonation, oxidation/reduction chemistry, and ionic and covalent bond energies. Ionization energies are closely related to the concepts of electronegativity, electron-richness, and the general reactivity of molecules. The energies and other characteristic features of the ionization bands observed in photoelectron spectroscopy provide some of the most detailed and specific quantitative information regarding the electronic structure and bonding in molecules. Photoelectron spectroscopy has served as a particularly important basis for the bonding models used to describe organic, inorganic, and organometallic molecules because the energetics of ion formation from the neutral ground state are directly related to orbital electron configurations, oxidation states, charge distributions, and covalency.
The Orbital Model of Ionization
Ionization is explicitly defined in terms of transitions between the ground state of a molecule and ion states as shown in Equation \ref{2} and as illustrated above. Information obtained from photoelectron spectroscopy is typically discussed in terms of the electronic structure and bonding in the ground states of neutral molecules, with ionization of electrons occurring from bonding molecular orbitals, lone pairs, antibonding molecular orbitals, or atomic cores. These descriptions reflect the relationship of ionization energies to the molecular orbital model of electronic structure.
Ionization energies are directly related to the energies of molecular orbitals by Koopmans' theorem, which states that the negative of the eigenvalue of an occupied orbital from a Hartree-Fock calculation is equal to the vertical ionization energy to the ion state formed by removal of an electron from that orbital, provided the distributions of the remaining electrons do not change. There are many limitations to Koopmans' theorem, but in a first order approximation each ionization of a molecule can be considered as removal of an electron from an individual orbital. The ionization energies can then be considered as measures of orbital stabilities, and shifts can be interpreted in terms of orbital stabilizations or destabilizations due to electron distributions and bonding. Koopmans' theorem is implicated whenever an orbital picture is involved, but is not necessary when the focus is on the total electronic states of the positive ions.
The direct ionization transition is not restricted by any symmetry selection rules because the ejected electron can carry any necessary angular momentum to make the process electric dipole allowed. Therefore, ionization to any excited positive ion state obtained by removal of a single electron and within range of the photon energy can be observed. That is, ionizations can be observed that correspond to removal of electrons from any of the occupied orbitals.
Photons with energies in the keV (X-ray) range are able to ionize down to the core electrons of atoms and molecules. Core ionizations of molecules are associated with the individual atoms present and fall in characteristic energy ranges related to the specific elements. The exact ionization energy of a core level is influenced by the charge potential around the atom and is useful for distinguishing between atoms in different chemical environments.
Information Content of Photoelectron Spectroscopy: While the energy information that is contained in a photoelectron spectrum is surely the most important information, further insight into the electronic structure of molecules is contained in other information within the spectrum. This includes Ionization Band Shape:

Removal of an electron changes the electronic structure and bonding in the molecule and results in a shift of the equilibrium internuclear separations. If the geometry changes are sufficiently great, the most probable (vertical) transitions from the neutral ground state to excited vibrational levels of the final ion state. When these vibrational levels are resolved, the change in vibrational spacing from the initial state to the final state gives a measure of the change in vibrational frequencies and force constants associated with the excitation, and the intensity pattern of the transitions to the excited vibrational levels (the Franck-Condon factors) gives a measure of the change in equilibrium bond distances.
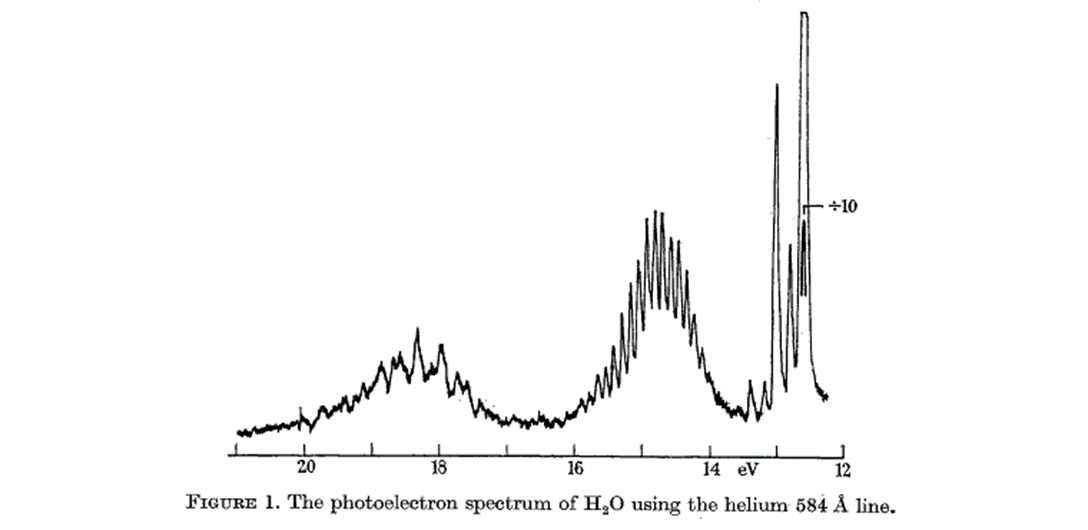
The two bands for water above indicated three orbital energies. Now, look at the MO for water, derived as a linear combination of SALC (for Hydrogens)
The oxygen atomic orbitals are labeled according to their symmetry as a1 for the 2s2 orbital and b2, a1 and b2 for 4 electrons in the 2p orbital. The two hydrogen 1s orbitals are premixed to form a A1 (bonding) and B2 (antibonding) MO.
- Notice that the O 1s orbital does not mix with the others - this is because it is much lower in energy than them.
- Notice also that the O 2px orbital does not mix with any others - this is because it is the only AO belonging to the b2 irrep. Electronic configuration of \(\ce{H2O}\):
\[\ce{1a_1^22a_1^21b_1^23a_1^21b_2^2} \nonumber \]
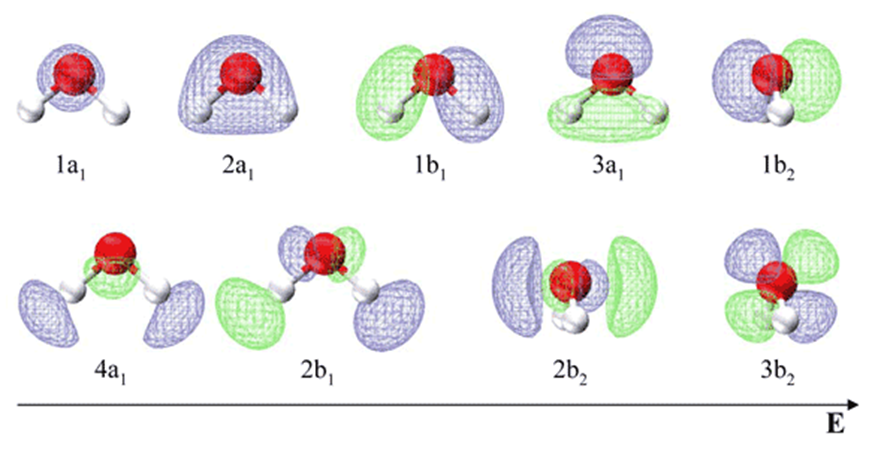
In agreement with this description the photoelectron spectrum for water shows two broad peaks for the 1b2 MO (18.5 eV) and the 2a1 MO (14.5 eV) and a sharp peak for the nonbonding 1b2 MO at 12.5 eV. This MO treatment of water differs from the orbital hybridization picture because now the oxygen atom has just one lone pair instead of two.
In this sense, water does not have two equivalent lone electron pairs as VSEPR suggests from general chemistry.

