1.8: Experiment 7 - Precipitation
- Page ID
- 255065
Learning Objectives
By the end of this lab, students should be able to:
- Describe precipitation reactions from the molecular perspective
- Record detailed observations for a reaction.
- Predict if a precipitate will form when combining two solutions.
- Predict when a chemical reaction will result in the formation of a gas.
- Write molecular, ionic, and net ionic equations for various reactions.
- Design an experiment to determine the concentration of a known solution of an ionic species utilizing solubility rules and stoichiometry.
- Calculate the concentration of a solution based on experimental (gravimetric) data.
Prior Knowledge:
Aqueous solution is any solution where water is present as a solvent. Rain, vinegar, orange juice are all examples of aqueous solutions that you come across in your everyday life. In chemistry aqueous solution indicated by adding "(aq)" to the reactant formula. For example, NaCl(aq) present as individual ions Na+ and Cl- dissolved in water. You might have heard that water is the universal solvent, however, water only dissolves substances that are hydrophilic (from the Greek "hydros" - water and "philia" - bonding or friendship). Compounds that do not dissolve in water remain a solid and indicated by "(s)". For example, AgCl(s).
Supplies
- Magnesium sulfate
- Calcium chloride
- Copper (II) Sulfate
- Sodium bicarbonate (baking soda)
- Vinegar (~5% acidity)
- Ammonia
- Scale
- Clean and dry test tubes
- Pipettes
- Cell phone with camera
- Laptop or computer with camera, speakers and microphone hooked up to internet
Solubility rules could be useful in our everyday life, but they are also extremely important in medicine. Sometimes doctors prescribe more than one solution to be administered by the intravenous (IV) route. Mixing two solutions that form a precipitate can lead to very serious consequences. For example, magnesium sulfate is used as an electrolyte replenisher or anticonvulsant, calcium chloride is indicated in the immediate treatment of hypocalcemic tetany (abnormally low levels of calcium in the body that cause muscle spasm) and intravenous sodium bicarbonate is a medication primarily used to treat severe metabolic acidosis. But what will happen if you mix them?
Procedure
Safety if you were to complete this lab in person:
- Obtain and wear goggles and gloves!!!
- Do non ingest any chemicals or inhale the vapors.
- Clean up all spills immediately! If contact with skin rinse with water for 15 minutes.
- Before proceeding with this or any other experiment students must sign the chemical lab safety form.
For each reaction in Part A and Part B record your observations, molecular equation, total ionic equation and net ionic equation. Make sure to write any evidence of a chemical reaction with sufficient detail to help you distinguish between similar precipitation reactions. Don't write “became cloudy” or “white solid”. Indicate if a gel is produced or crystals form, if the solid was powdery, etc. Keep in mind that some reactions will not occur and you should write NR (for No Reaction). You will know that a reaction occur if a precipitate, a gas, or color change occurred. Heat (whether it was consumed or evolved) can also be an indicator that reaction occurred, but you may not be able to tell in these videos.
Make sure to write any evidence of any evidence of a chemical reaction with sufficient detail to help you distinguish between similar precipitation reactions.
Week One
Reactions Worksheet
Part A. Precipitation Reaction Simulation
Using the simulation below, answer the questions on your worksheet.
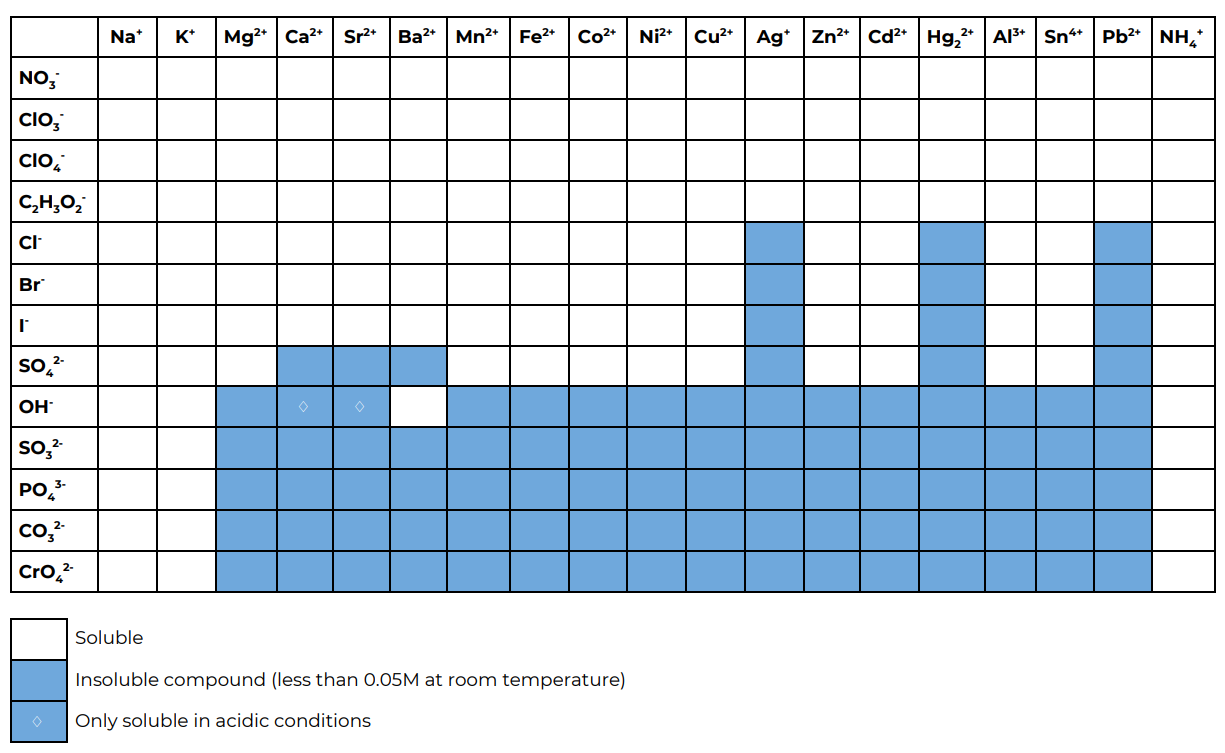
Part B. Predictions using the solubility table
Using this solubility table predict what happens in the reactions in part B.
Interactive Element
Group Worksheet
Before watching each video predict the outcome of the reaction (formation of gas (CO2 or H2) or precipitate, or no reaction). Then watch the video and on your worksheet write down 1) your observations, 2) molecular equation, 3) ionic equation and 4) net ionic equation for each reaction before moving to the next video.
1. Copper(II)* sulfate and sodium phosphate
* The video says Cu2SO4, but the reaction shown in this video is between copper (II) sulfate and sodium phosphate.
|
Query \(\PageIndex{2}\) |
2. Cadmium (II) chloride and sodium sulfide
|
Query \(\PageIndex{3}\) |
3. Nickel (II) chloride and sodium carbonate
|
Query \(\PageIndex{4}\) |
4. Lead (II) nitrate** and sodium sulfide
** The video says Pb2NO3, but the reaction shown is between lead (II) nitrate and sodium sulfide
|
Query \(\PageIndex{5}\) |
5. Magnesium and hydrochloric acid
|
Query \(\PageIndex{1}\) |
6. Nickel(II) chloride and sodium phosphate
|
Query \(\PageIndex{6}\) |
7. Silver nitrate and sodium carbonate
|
Query \(\PageIndex{7}\) |
Interactive Element
Week Two
Virtual Lab
Use the Gravimetric Analysis Virtual Lab for this assignment. Read Part I, then work in groups to complete Part II. Part III is your individual assignment and every group member will have their own unknown. You should receive an email from the TA with the unknown solution you need to use for the individual section of this assignment.
NOTE: If virtual lab says "Default Lab Setup" refresh your page
it should say: "Gravimetric Analysis of Unknown Lead Solutions"
Virtual Lab \(\PageIndex{1}\): ChemCollective Virtual Lab developed by Dave Yaron of Carnegie Mellon University, (http://www.chemcollective.org/). NOTE: if the lab loads as "Default Lab Setup" (with a bunch of acids, bases and indicators), refresh the page . You want it to load the "Gravimetric Analysis of Unknown Leas Solutions", which contains sodium chloride, potassium chromate and six unknowns.
Interactive Element
.
Interactive Element
Contributors and Attributions
-
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry) led the creation of this page for a 5 week summer course.
-
Elena Lisitsyna contributed to the creation and implementation of this page, including the generation of the HP5 question modules.
- Mark Baillie coordinated the modifications of this activity for implementation in a 15 week fall course, with the help of Elena Lisitsyna and Karie Sanford.

