5.2: Types of Chemical Reactions
- Page ID
- 281393
learning objectives
- List and define the 5 types of reactions based on the rearrangement of atoms
- Match given reactions to their respective category of reaction type
- Relate combustion reactions of hydrocarbons to climate change
- Relate decomposition reactions to production of hydrogen fuel cells.
Some Basic Types of Chemical Reactions
We are going to identify 5 basic "types" of chemical reactions because they have characteristic equations that allow us to use different techniques when we balance them. That is, in the next section when we balance a reaction, the first thing we will ask is what type of reaction it is, and then we will use the technique that is appropriate to that type reaction. There are other ways of classifying reactions that may actually tell us more about the chemistry, like are electrons transferred between atoms when a reaction occurs, which we will discuss later in this Chapter, and are called oxidation-reduction (redox) reactions, or acid base reactions (which at this level, occur when protons are transferred between between reactants).
- Formation Reactions (synthesis reactions)
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Combustion Reactions
Formation or Synthesis Reactions
H2(g) + Cl2(g) ® 2HCl(g)
As the name implies, two or more chemicals come together and form another chemical. Here, HCl is being formed from hydrogen and chlorine.
Decomposition Reaction
CaCO3(s) --> CaCO(s) + CO2(g)
As the name implies, something decomposes into something else. Here Calcium Carbonate decomposes to calcium oxide and carbon dioxide
Single Displacement Reactions
Fe(s) + CuSO4(aq) -> FeSO4(aq) + Cu(s)
F2(g) + BaCl2(aq) -> BaF2(g) + Cl2(g)
In a single displacement reaction something that is "pure" displaces something in a compound. As a rule of thumb, we say metals form cations and nonmetals from anions. So a metal can exist in two forms, as a pure metal, or as a cation (in an ionic compound). Likewise, a nonmetal can exist as something pure, or as an anion (in an ionic compound). In the first of the above two equations, the metal iron displaces the cation Copper(II) and we call that a metal single displacement reaction, while in the second, the fluorine displaces the anion chloride, and so we call that a nonmetal single displacement reaction. Note, sometimes these are called single replacement reactions.
If you note, these reactions involve a change in the charge of the metal or nonmetal, and so they involve the transfer of electrons. We will pick these up again later in this chapter, but a single displacement reactions involves the transference of electrons from the element that was pure to the element that was a cation, causing the cation to become pure, and the pure element to become a cation. If we just looked at the metals in the above equation we note that
Fe(s) + Cu+2(aq) -> Fe+2(aq) + Cu(s)
In this class we will focus on single displacement reactions involving metals, but in general chemistry you will need to understand these for both metals and nonmetals.
Metal Single Displacement
Fe(s) + AuSO4(aq) --> FeSO4(aq) + Au(s)
Some metals prefer to be ions, and other prefer to be pure. This can be understood by comparing iron ore with gold ore. Iron (pure) spontaneously rusts and iron ore that is mined must consists of compounds like hematite (Fe2O3) and magnetite (Fe3O4), while gold, a noble metal, does not tend to form ionic compounds, and so pure gold can be found in gold ore. So the above reaction is logical, in the sense that iron as a pure metal is not stable, while gold is.
Since the above reaction proceeds, we can correctly predict that:
FeSO4(aq) + Au(s) --> No Reaction
Metal Displacing Nonmetal
A metal can also displace hydrogen from an acid or even from water (look at water as H-OH, where the anion is hydroxide). For example
2Na(s) +2H2O(l) ->H2(g) + 2NaOH
Here the pure sodium metal becomes a cation, while a hydrogen is pulled off of each water to from hydrogen gas (a diatomic), and so hydrogen is behaving similar to a cation on the reactant side, but it is not a cation, it is an acidic hydrogen atom in a covalent bond. These reactions can give off heat which can then combust the hydrogen, as seen in Video \(\PageIndex{2}\).
Double Displacement Reactions
NiCl2(aq) + Na2CO3(aq) ® 2NaCl(aq) + NiCO3(s)
In a double displacement reaction, the ions of two ionic compounds swap counter ions. In this equation a precipitate formed
Nickle(II)chloride and sodium carbonate
Video \(\PageIndex{2}\): double displace reaction producing a preipitate
We will look at these in great detail in sections 5.3 and 5.4
Combustion Reaction
In a combustion reaction a compound reacts with oxygen and releases energy. Carbon dioxide and water are also released if the compound contains carbon.
CH4 + 2O2 --> CO2 + 2H2O + energy
Do combustion reactions always produce carbon dioxide and water?
No there are other types of combustion reactions, like the combustion of hydrogen and magnesium.
2H2 + O2 --> 2H2O + energy
2Mg + O2 -->MgO + energy
The hydrogen economy is considered as an alternative to fossil fuels and an area of intense current chemistry research. The concept is simple, one can use electricity generated by methods like solar, hydroelectric, wind, geothermal to split water into hydrogen and oxygen. Then you can transport the hydrogen to a new location and recombine them to produce energy where you need it, and produce no carbon dioxide. There are obvious challenges, like the storage of the hydrogen. As we shall learn later this semester, energy is the capacity to do work and transfer heat. So any energy lost as heat can not be used for work. You have probably heard of fuel cells, and they operate like a battery that consumes fuel, and can be more efficient than a combustion engine because they do not produce as much heat. In the following video the person uses a reversible fuel cell kit to demonstrate the concept of a hyrdogen economy. Simply speaking, you split water into hydrogen and oxygen when you are near a power source (solar, geothermal, wind, hydroelectric) then you recombine them where you want to use the energy, and in the process, you produce no carbon dioxide.
Practice Problems
Exercise \(\PageIndex{1}\)
Identify the types of reactions for each equation
- AlCl3(aq) + Na2CO3(aq) ® Al2(CO3)3(s) + NaCl(aq)
- C8H18 + O2 ® CO2 + H2O
- Li2S + Br2 ® 2LiBr + S
- H2CO3 --> H2O + CO2
- Ba(C2H3O2)2(aq) + 2HNO3(aq) ® Ba(NO3)2(aq) + 2HC2H3O2(aq)
- Answer a
-
Double Displacement
- Answer b
-
combustion
- Answer c
-
Single Displacement (non metal)
- Answer d
-
Decomposition
- Answer e
-
Double Displacement (involving acid)
Exercise \(\PageIndex{2}\)
Identify the types of reactions for each equation
- H2S(g) ® H2(g) + S(s)
- Pb + 2CuCl ® PbCl2 + 2Cu
- CH2CO(g) + 2 O2(g) ® 2 CO2(g) + H2O(g)
- HCl + NaOH --> NaCl + H2O
- Answer a
-
Decomposition
- Answer b
-
Single Displacement (metal displacement)
- Answer c
-
Combustion
- Answer d
-
Double Displacement (neutralization)
Predicting Products
For single displacement, double displacement and combustion reactions you need to be able to predict the products from the reactants.
Single Displacement
One of the reactant species is a pure elemental substance and the other is involved in a compound. If it is a metal displacement reaction the the cation in the compound becomes pure and the metal becomes a cation.
\[\textcolor{red}{Metal} +\underbrace{ \textcolor{blue}{Cation_aAnion_b}}_{\text{(neutral formula of ionic compound)}} \rightarrow \; \textcolor{blue}{Metal} \underbrace{ \textcolor{red}{Cation_x}\textcolor{blue}{Anion_y}}_{\text{(neutral formula of ionic compound)}}\]
\[\textcolor{red}{Al} + \textcolor{blue}{FeSO_4} \rightarrow \; ? \]
Aluminum only has one ion, Al+3 and so the product salt is the neutral salt between Al+3 and SO4-2, Al2(SO4)3.
\[\textcolor{red}{Al} + \textcolor{blue}{FeSO_4} \rightarrow \; \textcolor{blue}{Fe} + \textcolor{red}{Al}_2(\textcolor{blue}{SO_4})_3 \\ \text{(unbalanced equation)} \]
Exercise \(\PageIndex{3}\)
\[\textcolor{red}{Al} + \textcolor{blue}{Fe}_2\textcolor{blue}{(SO_4)}_3 \rightarrow \; ? \]
- Answer
-
\[\textcolor{red}{Al} + \textcolor{blue}{Fe}_2\textcolor{blue}{(SO_4)}_3 \rightarrow \; \textcolor{blue}{Fe} + \textcolor{red}{Al}_2(\textcolor{blue}{SO_4})_3 \\ \text{(unbalanced equation)} \]
Note, iron is type II metal and forms more than one stable ion, while Aluminum is type one and is always [+3], and so the products are the same as the above illustration.
Double Displacement
For double displacement you swap counterions.
\[\underbrace{ \textcolor{blue}{Cation_aAnion_b}}_{\text{(neutral formula of ionic compound)}}+\underbrace{ \textcolor{red}{Cation_cAnion_d}}_{\text{(neutral formula of ionic compound)}} \rightarrow \underbrace{ \textcolor{blue}{Cation_w}\textcolor{red}{Anion_x}}_{\text{(neutral formula of ionic compound)}} +\underbrace{ \textcolor{red}{Cation_y}\textcolor{blue}{Anion_z}}_{\text{(neutral formula of ionic compound)}}\]
Strategy
1.Identify Reactant Ions and their Charge
2. Swap Ions
3. Use Principle of Charge Neutrality to determine formula of products
Example \(\PageIndex{1}\)
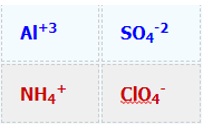
\[\textcolor{blue}{Al}_2(\textcolor{blue}{SO_4})_3 + \textcolor{red}{NH_4ClO_4} \rightarrow \, ?\]
Solution
1. Identify reactant ions and their charge
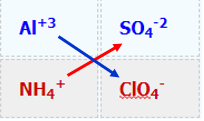
- Swap Ions
3. Using Principle of Charge Neutrality to Identify Products
\[\textcolor{blue}{Al}_2(\textcolor{blue}{SO_4})_3 + \textcolor{red}{NH_4ClO_4} \rightarrow \, \textcolor{blue}{Al}(\textcolor{red}{ClO_4})_3 +(\textcolor{red}{NH_4})_2\textcolor{blue}{SO_4} \\ \text{(unbalanced equation)}\]
Combustion
For carbon based compounds the products of complete combustion are carbon dioxide and water.
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- November Palmer & Emily Chaote (UALR)
- Vincent Belford (H5P)
- Ronia Kattoum (Learning Goals)


