4.1: Chemical Compounds
- Page ID
- 279912
learning objectives
- Differentiate between ionic compounds and molecular compounds in the way the bonds form and varying characteristics
- Identify elements that exist as diatomic molecules
- Define allotropes
- Interpret different types of formulas including molecular formulas, structural formulas, and condensed structural formulas
Introduction
Chemical compounds occur when 2 or more atoms are bonded together. We will consider two basic types of bonds, covalent and ionic, and thus focus on two basic kinds of chemical compounds, covalent and ionic. Each of these compounds is associated with a chemical formula, and it is important to note that there is a third type of bond that is very common and which we are not considering here, and that is the metallic bond. A pure metal, or an alloy like Brass, (a mixture of copper and zinc) is neither an ionic or covalent compound. Brass does not form a "compound" in the classical sense of Dalton's theory, where there is a constant ratio of copper to zinc that leads to a specific formula. In fact brass can be considered to be a solid solution, and the different grades of brass depend on the ratio of copper to zinc. So we do not associate a typical chemical formula with metals and alloys.
Chemical bonds are the result of Coulombic (electrostatic) interactions between charged particles, where like charges repel and opposite charges attract. In the case of covalent bonds, these are between electrons and the nuclei of one atom with the electrons and nuclei of another. In the case of ionic bonds, these are between cations ([+] charged ions) and anions ([-] charged.
Types of Bonds
There are actually 4 types of bonds that commonly occur, covalent, ionic, polar covalent and metallic. At this point in our study we will focus on two types, covalent and ionic. The type of bond that forms between two atoms depends on the type of atoms and can often be determined by identifying if the bonding atoms are metallic or covalent. So we should start with figure 3.5.4 of the last chapter, which we are reposting below as figure \(\PageIndex{1}\).
Covalent Molecules
Covalent Bonds occur between two nonmetals and involving a sharing of valence (outer shell) electrons. These are the classical molecules and can range from simple molecules like water to very complex molecules like proteins. We will cover these in section 2 of this chapter. You will be responsible for naming simple covalent compounds (last section of this chapter). The molecular formula is the formula of the discrete entity of matter that a covalent compound represents. So H2O represents a water molecule where the entity of matter is two hydrogens bonded to one oxygen.
Ionic Compounds
Ionic compounds occur between a metal and a nonmetal, and involving the formation of ions. The metal gives an electron to the nonmetal, and the positive charge metal is called a cation and the negative charged nonmetal is called an anion
- Cation: Net positive charge, the number of protons is greater than the number of electrons
- Anion: Net negative charge, the number of protons is less than the number of electrons.
Although true, the above statement that ionic compounds form between metals and nonmetals is a bit simplistic, and not all ionic compounds a formed between metals and nonmetals, but it is a good rue of thumb if you have a compound between two different elements. As we will see later in this chapter, some ionic bonds involve polyatomic ions, which are covalently bonded molecules that have a net charge.
Ionic compounds form crystal lattice structures in three dimensional space where the geometry is a result of the attraction between the opposite charged ions and the repulsion between the like charged ions, and this structure can extend in space until all the available ions are used up. If you look at a salt crystal you will see that each positive ion is surrounded on all 6 sides by negative ions, and that a crystal lattice forms. So it makes no sense to have a "molecular formula" for an ionic compound, because the number of atoms will grow as the crystal grows.
-
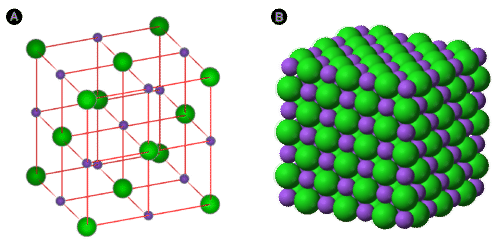

Figure \(\PageIndex{2}\) On the left is a crystal lattice of NaCl table salt, which is also shown on the right, where it forms Halite crystals. Because the number of ions depends on the size of the crystal, we use the lowest ratio of positive to negative ions that results in a neutral structure.
Principle of Charge Neutrality
This is a very important concept, which states that the formula of an ionic compound must be neutral as there were a net charge, the repulsive forces between the like charged ions would be greater in number than the attractive forces between opposite charged ions and so the material woud never form a stable structure. If we look at figure \(\PageIndex{2}\) we see that each sodium ion is within the bulk of the cube is bonded to 6 chloride ions, while each chloride ion is bonded to each sodium, and the total number of sodium and chloride ions is a consequence of the size of the crystal and so there is no discrete formula that describes sodium chloride the way a molecular formula describes a molecule like water. As a consequence we use the lowest whole number ratio of cations ([+] ions) to anions ([-] ions) to describe an ionic compound and the formula of sodium chloride is NaCl and not Na6Cl6. That is, the formula is the lowest whole number ratio of cations to anions that results in a neutral structure, and not a representation of the actual strucute itself.
Polar Covalent Compounds
Polar covalent compounds are covalent compounds with unequal sharing of the bonding electrons. They can behave very differently than pure covalent compounds that have equal sharing of the bonding electrons. Acids are a type of polar covalent compound and the following video shows the difference between covalent, ionic and polar covalent compounds. Please note the video goes over some concepts we will study later (electronegativity, and polar bonds), but if you watch between eleven seconds and 49 seconds you get a good visualization of the difference between a ionic and covalent bond.
Metallic Bonds
Metallic bonds occur between metals. If the metals are different, they are called alloys. Bonze is an alloy of copper and tin, while brass is an alloy of copper and zinc, and the properties of the alloys can vary as the ratio of the different metals varies. What is important to know is that metals form two types of bonds, metallic bonds between metals and ionic bonds with nonmetals. The iron of a steel fence is a metallic bond, and the rust on the iron, which forms when it reacts with the nonmetal oxygen, is an ionic bond. It should be noted that rust is very complex and there is no single formula that describes it
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Anonymous
- Modifications by Ronia Kattoum
- Elena Lisitsyna (H5P interactive modules)

