3.1: The Nuclear Atom
- Page ID
- 278748
The Nuclear Atom- Overview
The precise physical nature of atoms finally emerged from a series of elegant experiments carried out between 1895 and 1915. The most notable of these achievements was Ernest Rutherford's famous 1911 alpha-ray scattering experiment, which established that
- Almost all of the mass of an atom is contained within a tiny (and therefore extremely dense) nucleus which carries a positive electric charge whose value identifies each element and is known as the atomic number of the element.
- Almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric charge, reside. The extremely small mass of the electron (1/1840 the mass of the hydrogen nucleus) causes it to behave as a quantum particle, which means that its location at any moment cannot be specified; the best we can do is describe its behavior in terms of the probability of its manifesting itself at any point in space. It is common (but somewhat misleading) to describe the volume of space in which the electrons of an atom have a significant probability of being found as the electron cloud. The latter has no definite outer boundary, so neither does the atom. The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. Atomic radii are typically 30-300 pm.
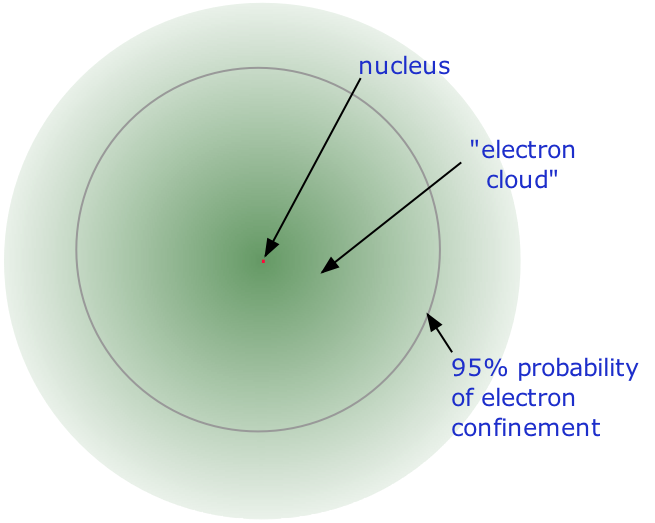
The nucleus is itself composed of two kinds of particles. Protons are the carriers of positive electric charge in the nucleus; the proton charge is exactly the same as the electron charge, but of opposite sign. This means that in any [electrically neutral] atom, the number of protons in the nucleus (often referred to as the nuclear charge) is balanced by the same number of electrons outside the nucleus. The other nuclear particle is the neutron. As its name implies, this particle carries no electrical charge. Its mass is almost the same as that of the proton. Most nuclei contain roughly equal numbers of neutrons and protons, so we can say that these two particles together account for almost all the mass of the atom.
Note
Because the electrons of an atom are in contact with the outside world, it is possible for one or more electrons to be lost, or some new ones to be added. The resulting electrically-charged atom is called an ion.
Interactive Slide Show
The following slide show covers the history of the development of atomic theory from early Greek philosophers to modern atomic theory (click the arrow on the right). Prep Chem students will not be tested on this material, but they will need to know it in general chemistry, and interested students are advised to check out the general chemistry section 2.1.2 and 2.1.3.
Subatomic Particles
Atoms consist of electrons, protons, and neutrons. Although this is an oversimplification that ignores the other subatomic particles that have been discovered, it is sufficient for discussion of chemical principles. Some properties of these subatomic particles are summarized in Table \(\PageIndex{1}\), which illustrates three important points:
- Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton, respectively.
- Neutrons have approximately the same mass as protons but no charge. They are electrically neutral.
- The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms.
| Particle | Mass (g)* | Atomic Mass (amu)* | Electrical Charge (coulombs) | Relative Charge |
|---|---|---|---|---|
| electron | 9.1093837015×10−28 | 0.0005485799090 | −1.602176634 × 10−19 | −1 |
| proton | 1.67262192369 ×10−24 | 1.007276466621 | +1.602 × 10−19 | +1 |
| neutron | 1.67492749804×10−24 | 1.00866491595 | 0 | 0 |
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Anonymous
- Modified by Joshua Halpern, Scott Sinex and Scott Johnson
- November Palmer & Ronia Kattoum(UALR)
- Elena Lisitsyna (H5P interactive modules)

