1.3: Chemical Safety
- Page ID
- 290467
General Information
Chemical Safety is usually covered in the chemistry laboratory and we will not be doing any experiments in this class. It is still important to discuss safety and go over some resources that are available to the public. One of the goals of this section is to provide you resources for chemical safety in the digital age, when it is so easy to find misinformation. We will also discuss the evaluation of safety information, which is ever so important now, in the time of COVID-19, and this material is sort of separate from the rest of the class, and will not be examined on, but is here for your benefit. So lets start with workplace safety, and the Chemical Hygiene Plan at UALR.
Chemical Hygiene Plan
Every university has a CHP (Chemical Hygiene Plan) as required by OSHA standard 29 CFR 1910.1450 and a CHO (Chemical Hygiene Officer) who is responsible for its implementation, and UALR's CHP can be found at the Facilities Management Web Page. Within the CHP are a set of standard SOPs (Safe Operating Procedures) that represent the minimum safe practices for the handling of hazardous chemicals and operation of equipment (like how to secure a compressed gas tank). Every research lab at the university is required to develop and maintain SOPs for the laboratory practices that are performed within their labs, and here is a link to the template for developing laboratory specific SOPs. The CHP also defines the PPE (Personal Protective Equipment) needed to perform work in a laboratory, and in the university teaching laboratory the instructor would be responsible for ensuring students abide by the established SOPs, and wear proper PPE, like safety glasses and closed toe shoes (no flip-flops in the chemistry laboratory), and do not perform any unauthorized experiments.
Chemical Safety Resources
Prudent Practices
The National Research Council of the National Academies of Sciences has published a book "Prudent Practices in the Laboratory" that can be downloaded for free and has a wealth of information on chemical safety, including a copy of OSHA's Laboratory Standard (29 CFR 1910.1450). There is also an accompanying zip file of a CD that contains Laboratory Chemical Safety Summaries (LCSS) and additional information. LCSS are very useful and in a bit we will look at the PubChem LCSS that are freely available over the web.

Do you know who is considered the founder of the National Academy of Science? Check it out on Wikipedia! Yes, a president of the United States is considered to be a co-founder of the National Academy of Science and it was created by an Act of Congress to "provide independent, objective advice to the nation on matters related to science and technology...." (see Wikipedia article). But providing information on chemical safety can be very complicated, why before 2012 we had different symbols for chemicals depending on if they were being transported (DOT regulations), used (OSHA regulations) or being disposed of (EPA regulations), and of course, we live in a global world where chemicals are shipped across borders, and so there are not only different laws, but different languages.
UN GHS
What a mess! So the United Nations put forth a system, the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) and the OSHA's laboratory standard is actually integrated into the United States implementation of the UN GHS. The 8th edition can be downloaded as a PDF for free and you can find codes that mean the same thing in any country that adheres to the UN recommendations. So if a chemical is made in China, travels through Indonesia, Saudi Arabia, Mexico and is used in the United States, everyone knows how to read the hazard codes. Another component of the GHS is the Safety Data Sheets (SDS), which provide hazard information outlined in figure \(\PageIndex{2}\) and are required for every chemical that is manufactured or enters the US. So if you want to find information on a product, go find the SDS, and further information bay be obtained at Safety Emporium.


And interesting thing about the UN GHS is they allow us to compare the laws of different nations. That is, the laws of a country determine what codes must be applied to a chemical, and since they all use the same code, you can quickly compare the laws. So when labeling a chemical you still have to take into consideration what countries that chemical will be in, but one label works in them all (this is similar to the air pollution laws we are all familiar with, and how gasoline engines in machines like lawn mowers state if they are compliant with California regulations, even if they are sold in Arkansas). So the safety information associated with a chemical is influenced by the laws of the country which regulate the labeling of that chemical, and you should be prepared to look further.
PubChem LCSS
The National Institute of Health's (NIH) National Library of Medicine's (NLM) PubChem have developed LCSS that model the LCSS of the NRC, but extract data from multiple chemical compound databases. As of January 2021 there are LCSS for 150,8633 chemical substances that can be obtained through PubChem (not, the actual number will change over time and if you click that link you can both access the LCSS and see how many there are). This is a very valuable resource for finding safety information on chemicals and it is structured so you can understand where the information comes from. Why not take a minute to scroll through the LCSS of potassium permanganate, and there really is a lot of info there. Now to get a bearing on how complicated safety information can be, look at section 7 (First Aid), and note the first statement says Induce vomiting, but then in section 7.4 it says do not induce vomiting. Can you figure that out? Each of those statement provides links to the original source, and one was a solid that was inhaled, while the other was a liquid that was drunk.
COVID-19 Pandemic Issues
This course is being taught online because of the COVID-19 pandemic and there is a lot of conflicting information being spread across the internet, which makes it an ideal topic for discussing safety. In fact PPE that are normally used in the lab like masks and face shields are now often required in public. Students are expected to follow CDC guidelines when in public, which includes covering your mouth and nose when in public and it is worth watching this video from Florida Atlantic University and then discussing the kinds of PPE one should use to prevent either contact or air born spread of COVID-19.
Video \(\PageIndex{2}\): https://youtu.be/MMwYsGews-8
In the following video a chemistry professor at UC-Boulder who studies aerosol transmission of COVID-19 discusses the difference between water droplets and aerosols, while the video goes over some of the issues with evaluating online information. Later this semester we will learn more about aerosols, which are an interesting type of mixture called a colloidal dispersion. Aerosols are sort of a like mixture between a liquid and a gas, and the liquid components can absorb many chemical compounds but like a gas, travel great distances. Many so called "gas phase reactions" actually occur in aerosols and this are very important in fields like atmospheric chemistry.
Video \(\PageIndex{2}\): https://youtu.be/Y3zKRHzccMk
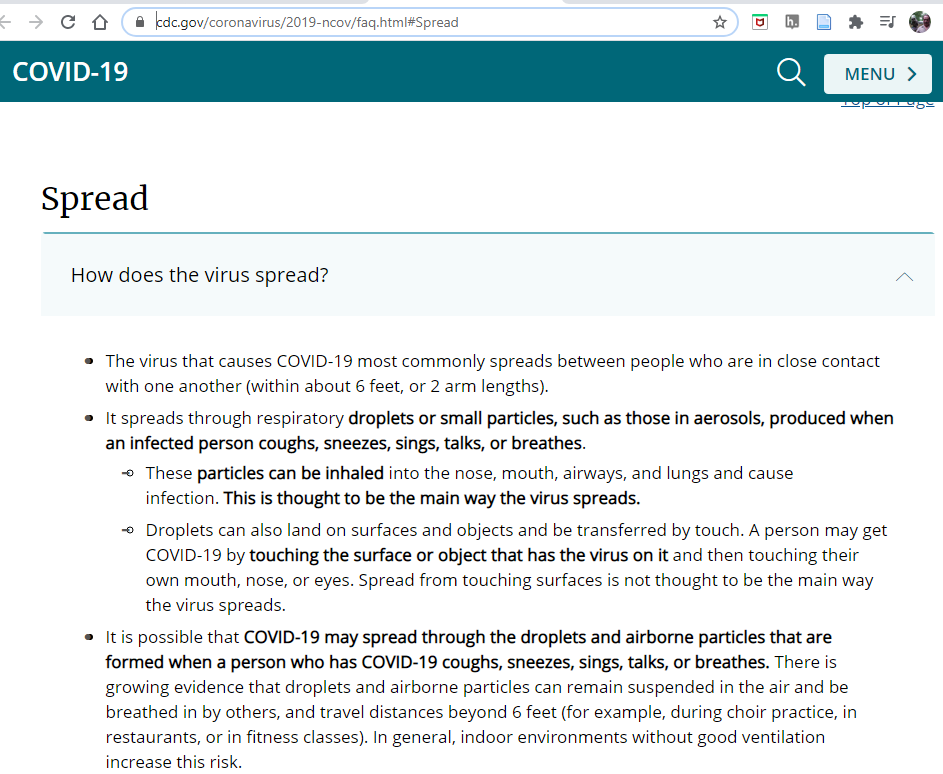
On Sep 21, 2020 the above video describes how the CDC posted an alert that the primary mechanism for the spread of COVID-19 was through aerosols and than strangly took that recommendation down saying it was posted in error, which can lead one to wonder what was going on with the CDC? Fast forward to Jan. 19, 2021 and the screen capture below clearly states that aerosols are involved in the spread of COVID-19. The important thing with regards to safety is to get all the information you can, assess where that information is coming from, and then make up your mind. Safety literacy is more than finding information on the web, it is also evaluating that information, and in the case of COVID-19, that could be the difference between life and death.
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Elena Lisitsyna

