3.5.2: Exercises
- Page ID
- 165281
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)___ 1. What is the possible identity of enzyme 2?
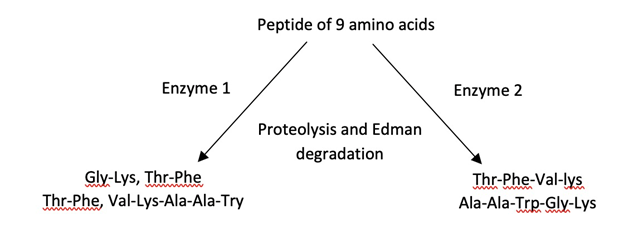
a. papain
b. cyanogen bromide
c. pepsin
d. trypsin
e. chymotrypsin
___ 2. Which of the following techniques may be used to determine the tertiary structure of a protein?
a. X-ray crystallography
b. HPLC
c. ELISA
d. 1D proton NMR
e. mass spectrometry
___3. One liter of an aqueous solution contains 0.10 M aspartate at pH= pI, and 0.05 M NaOH is subsequently added. What is the pH after adding NaOH?
a. 3.1
b. 3.6
c. 4.1
d. 6.2
e. 8.0
___ 4. Which of the following amino acids would most likely be buried in the interior of a water-soluble protein?
a. Arg
b. Thr
c. Trp
d. Glu
e. His
___ 5. Which of the following described the quaternary structure of a protein?
a. helix or sheet stabilized by hydrogen bonds
b. formation of all four kinds of noncovalent bonds
c. a multi-peptide structure
d. requires disulfide bonds
e. none of the above are correct
___6. Somatostatin, a peptide hormone which inhibits the release of pituitary growth hormone, has the sequence:
___ 7. Which of the following agents is expected to alter the covalent structure of somatostatin?
a. SDS
b. heat to 100 oC
c. urea
d. cyanogen bromide
e. none of the above
___8. Which technique uses the attraction of the protein for a particular chemical group during protein purification?
a. affinity chromatography
b. gel-filtration chromatography
c. HPLC
d. SDS PAGE
e. salting out
___9. The AIGFRLKT was treated with the enzyme chymotrypsin. Which of the following could best separate the resulted peptides?
a. gel-filtration chromatography
b. affinity chromatography
c. ion-exchange chromatography
d. all of the above
e. none of the above
___10. You have purified a protein. Determination by gel-filtration chromatography yields 50 kDa, but mass spectrometry (MS) yielded a 25-kDa species. When the protein was per-treated with beta-mercaptoethanol followed MS determination, a single molecular species of 12.5 kDa showed up. Which of the following properly describes the structure of the protein? Hints: a dimer contains two subunits and a tetramer contains four subunits; each subunit is a separate polypeptide chain
a. a dimer of 25 kDa subunits connected by disulfide bonds
b. a tetramer of 12.5 kDa subunits with all subunits linked to each other by disulfide bonds
c. a tetramer of 12.5 kDa subunits with two subunits forming 25 kDa dimers using disulfide bonds
d. a tetramer of 25 kDa subunits linked by disulfide bonds
___11. What are some of the modifications that proteins acquire?
a. cleavage of the protein
b. carboxylation
c. phosphorylation
d. all of the above
___12. Which of the following amino acids would most likely be found in the triple helix of collagen?
a. Asp
b. His
c. Phe
d. Gln
e. Gly
___13. Which atom(s) in proteins are regular hydrogen-bond acceptors?
a. carbon
b. oxygen
c. nitrogen
d. sulfur.
e. oxygen and nitrogen
14. You plan to purify and subsequently characterize a native protein.
a. List 4 techniques for purification of your protein (not denatured).
b. After purification, you would like to know the purity of your protein. List one technique you may use.
c. Now you would like to know the 3-D structure of the protein. List 2 techniques you may use.

