9.2 Gluconeogenesis: Reaction and regulation
- Page ID
- 166218
Introduction
The need for energy is important to sustain life. Organisms have evolved ways of producing substrates required for the catabolic reactions necessary to sustain life when desired substrates are unavailable. The main source of energy for eukaryotes is glucose. When glucose is unavailable, organisms are capable of metabolizing glucose from other non-carbohydrate precursors. The process that coverts pyruvate into glucose is called gluconeogenesis.
Gluconeogenesis Is Not a Reversal of Glycolysis
In glycolysis, glucose is converted into pyruvate; in gluconeogenesis, pyruvate is converted into glucose. However, gluconeogenesis is not a reversal of glycolysis. Several reactions must differ because the equilibrium of glycolysis lies far on the side of pyruvate formation. The actual ΔG for the formation of pyruvate from glucose is about -20 kcal mol-1 (-84 kJ mol-1) under typical cellular conditions. Most of the decrease in free energy in glycolysis takes place in the three essentially irreversible steps catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase.
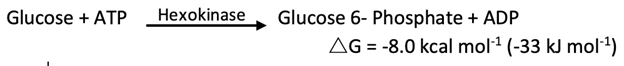


In gluconeogenesis, the following new steps bypass these virtually irreversible reactions of glycolysis:
1. Phosphoenolpyruvate is formed from pyruvate by way of oxaloacetate through the action of pyruvate carboxylase and phosphoenolpyruvate carboxykinase.
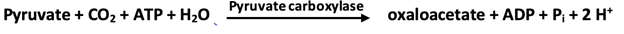
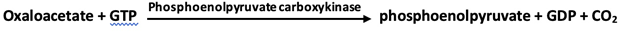
2. Fructose 6-phosphate is formed from fructose 1,6-bisphosphate by hydrolysis of the phosphate ester at carbon 1. Fructose 1,6-bisphosphatase catalyzes this exergonic hydrolysis.
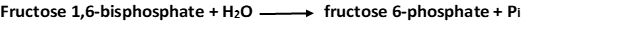
3. Glucose is formed by hydrolysis of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphatase.
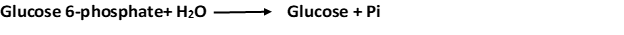
Regulation
It is important for organisms to conserve energy, they have derived ways to regulate those metabolic pathways that require and release the most energy. In glycolysis and gluconeogenesis seven of the ten steps occur at or near equilibrium. In gluconeogenesis the conversion of pyruvate to glucose all occur very spontaneously which is why these processes are highly regulated. It is important for the organism to conserve as much energy as possible. When there is an excess of energy available, gluconeogenesis is inhibited. When energy is required, gluconeogenesis is activated.
- The conversion of pyruvate to PEP is regulated by acetyl-CoA. Because acetyl-CoA is an important metabolite in the TCA cycle which produces a lot of energy, when concentrations of acetyl-CoA are high organisms use pyruvate carboxylase to channel pyruvate away from the TCA cycle. If the organism does not need more energy, then it is best to divert those metabolites towards storage or other necessary processes.
- The conversion of fructose-1,6-bisphosphate to fructose-6-phosphate with the use of fructose-1,6-phosphatase is negatively regulated and inhibited by the molecules AMP and fructose-2,6-bP. These are reciprocal regulators to glycolysis' phosphofructokinase. Phosphofructosekinase is positively regulated by AMP and fructose-2,6-bP. Once again, when the energy levels produced are higher than needed, i.e. a large ATP to AMP ratio, the organism increases gluconeogenesis and decreases glycolysis. The opposite also applies when energy levels are lower than needed, i.e. a low ATP to AMP ratio, the organism increases glycolysis and decreases gluconeogenesis.
- The conversion of glucose-6-P to glucose with use of glucose-6-phosphatase is controlled by substrate level regulation. The metabolite responsible for this type of regulation is glucose-6-P. As levels of glucose-6-P increase, glucose-6-phosphatase increases activity and more glucose is produced. Thus glycolysis is unable to proceed.
Importance
This metabolic pathway is important because the brain depends on glucose as its primary fuel and red blood cells use only glucose as a fuel. The daily glucose requirement of the brain in a typical adult human being is about 120 g, which accounts for most of the 160 g of glucose needed daily by the whole body. The amount of glucose present in body fluids is about 20 g, and that readily available from glycogen, a storage form of glucose, is approximately 190 g. Thus, the direct glucose reserves are sufficient to meet glucose needs for about a day. During a longer period of starvation, glucose must be formed from noncarbohydrate sources.
The major site of gluconeogenesis is the liver, with a small amount also taking place in the kidney, brain, skeletal muscle, or heart muscle.
References
Garrett, H., Reginald and Charles Grisham. Biochemistry. Boston: Twayne Publishers, 2008.
Raven, Peter. Biology. Boston: Twayne Publishers, 2005.

