10.2: Electrochemistry Lab
- Page ID
- 357183
This is the last lab of the semester and there is no formal lab report. Instead this is an exploratory lab where you will design an experiment using electrochemistry to determine the concentration of an unknown solution of copper(II). All work is to be handed in at the end of the lab and you need to study for the lab final for next week.
Learning Objectives
Goals:
- Design an experiment using an electrochemical cell to determine the concentration of an unknown copper (II) solution
By the end of this lab, students should be able to:
- Be able to design an experiment where they can calculate Eocell.
Prior knowledge:
- Single Displacement Reactions (section 3.2.1.2)
- Activity Series (section 3.6.2)
Concurrent Reading:
- Electrochemical Cells (section 19.3)
Safety
- Emergency Preparedness
- Eye protection is mandatory in this lab, and you should not wear shorts or open toed shoes.
- Any spills should be cleaned up immediately
- Minimize Risk
- Check t
- Recognize Hazards
- You
Equipment and materials needed
| 1M ZnSO4(aq) | metal strip of zinc | 1M CuSO4(aq) |
| metal strip of copper | unknown copper solution | volt meter |
| spot plates | filter paper |
Background
Read section 19.3 of your text before proceeding with this lab. We will look at the spontaneous reaction of zinc metal with copper (II) solutions, for which the net ionic equation is:
\[Zn(s) + Cu^{+2} \rightarrow Cu(s) + Zn^{+2} \]
The Daniel Cell takes advantage of this spontaneous reaction and figure \(\PageIndex{1}\) shows the basic components of an electrochemical cell based on this reaction.
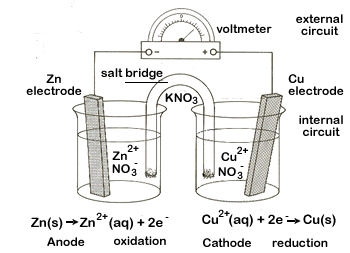
The basic components of a galvanic electrochemical cell.
- Anode, where oxidation occurs (the more active metal, the one with the lower standard state reduction potential)
- Cathode, where reduction occurs (the less active metal, the one with the higher standard reduction potential)
- External circuit (here is is a voltmeter, which has a high resistance and measures the electric potential. This could be a load like a light bulb or motor, in which case work could be done as the current flows through the circuit)
- Salt bridge (these are counter ions that prevent the build up of charge that would stop the current from flowing. They also need to stop the migration of the copper (II) to the zinc strip, then electrons would transfer directly to the copper ion without ever flowing through the external circuit).
In shorthand notation (section 19.3.3) and at standard state conditions the Daniell Cell would be written as:
\[Zn(s)|Zn^{+2}(1M)||Cu^{+2}(1M)|Cu(s)\]
The Nernst Equation (section 19.7) for an electrochemical cell relates the electric potential at any concentration to that at standard state concentrations.
\[E=E° -\frac{RT}{nF} \ln Q\]
Where,
- E= Cell potential at any concentration
- Eo= Cell potential at standard state concentrations (1M)
- R=Ideal gas constant (8.314J/mol-K)
- T=Absolute Temperature (K)
- n=moles electrons transferred in balanced redox reaction
- F=Faraday's constant (96,500J/V-mole e-)
- Q=Reaction Quotient of redox reaction \( \left (\frac{[Zn^{+2}]}{[Cu^{+2}]} \right )\) for the above cell
So for this reaction the Nernst Eq. becomes:
\[E=E° -\frac{RT}{nF} \ln \left (\frac{[Zn^{+2}]}{[Cu^{+2}]} \right )\]
If we know Eocell and the concentration of one species, we can determine the concentration of the other by measuring Ecell. Here we solve for [Zn+2].
\[[Zn^{+2}]=[Cu^{+2}]e^{\frac{nF}{RT}\left ( E_{cell}^{o}-E_{cell} \right )}\]

