Symmetry and Chirality
Molecules that are nonsuperimposable mirror images of each other are said to be chiral (pronounced “ky-ral,” from the Greek cheir, meaning “hand”). Examples of some familiar chiral objects are your hands. Your left and right hands are nonsuperimposable mirror images. (Try putting your right shoe on your left foot—it just doesn’t work.) An achiral object is one that can be superimposed on its mirror image, as shown by the superimposed flasks in the figure below.
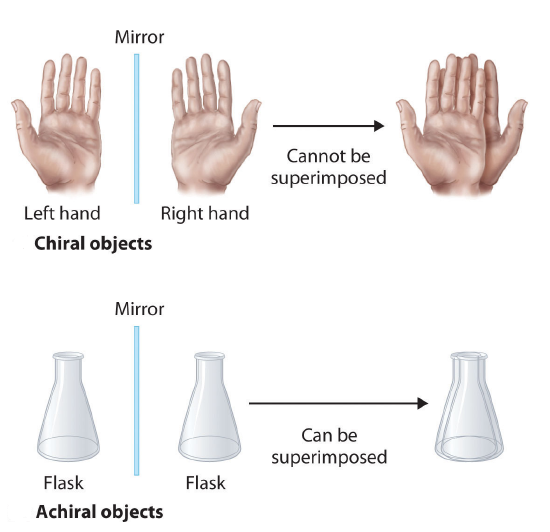
An an important questions is why is one chiral and the other not? The answer is that the flask has a plane of symmetry and your hand does not. A plane of symmetry is a plane or a line through an object which divides the object into two halves that are mirror images of each other. When looking at the flask, a line can be drawn down the middle which separates it into two mirror image halves. However, a similar line down the middle of a hand separates it into two non-mirror image halves. This idea can be used to predict chirality. If an object or molecule has a plane of symmetry it is achiral. If if lacks a plane of symmetry it is chiral.
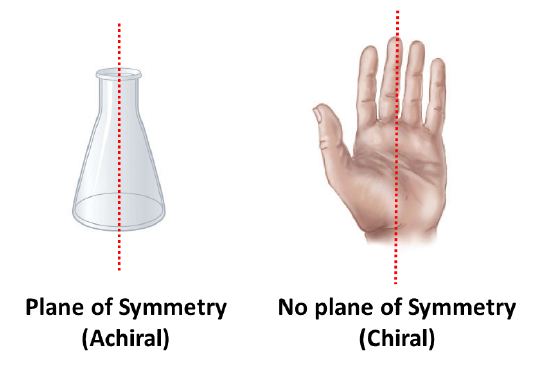
Symmetry can be used to explain why a carbon bonded to four different substituents is chiral. When a carbon is bonded to fewer than four different substituents it will have a plane of symmetry making it achiral. A carbon atom that is bonded to four different substituents loses all symmetry, and is often referred to as an asymmetric carbon. The lack of a plane of symmetry makes the carbon chiral. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.
Identifying Chiral carbons
Identifying chiral carbons in a molecule is an important skill for organic chemists. The presence of a chiral carbon presents the possibility of a molecule having multiple stereoisomers. Most of the chiral centers we shall discuss in this chapter are asymmetric carbon atoms, but it should be recognized that other tetrahedral or pyramidal atoms may become chiral centers if appropriately substituted. Also, when more than one chiral center is present in a molecular structure, care must be taken to analyze their relationship before concluding that a specific molecular configuration is chiral or achiral. This aspect of stereoisomerism will be treated later. Because an carbon requires four different substituents to become asymmetric, it can be said, with few exceptions, that sp2 and sp hybridized carbons involved in multiple bonds are achiral. Also, any carbon with more than one hydrogen, such as a -CH3 or -CH2- group, are also achiral.
Looking for planes of symmetry in a molecule is useful, but often difficult in practice. It is difficult to illustrate on the two dimensional page, but you will see if you build models of these achiral molecules that, in each case, there is at least one plane of symmetry, where one side of the plane is the mirror image of the other. In most cases, the easiest way to decide whether a molecule is chiral or achiral is to look for one or more stereocenters - with a few rare exceptions, the general rule is that molecules with at least one stereocenter are chiral, and molecules with no stereocenters are achiral.
Determining if a carbon is bonded to four distinctly different substituents can often be difficult to ascertain. Remember even the slightest difference makes a substituent unique. Often these difference can be distant from the chiral carbon itself. Careful consideration and often the building of molecular models may be required. A good example is shown below. It may appear that the molecule is achiral, however, when looking at the groups directly attached to the possible chiral carbon, it is clear that they all different. The two alkyl groups are differ by a single -CH2- group which is enough to consider them different.

Example \(\PageIndex{1}\)
Predict if the following molecule would be chiral or achiral:

- Answer
-
Achiral. When determinig the chirality of a molecule, it best to start by locating any chiral carbons. An obvious candidate is the ring carbon attached to the methyl substituent. The question then becomes: does the ring as two different substituents making the substituted ring carbon chiral? With an uncertantity such as this, it is then helpful try to identify any planes of symmetry in the molecule. This molecule does have a plane of symmetry making the molecul achiral. The plane of symmetry would be easier see if the molecule were view from above. Typically, monosubstitued cycloalkanes have a similar plane of symmetry making them all achiral.

Exercise 5.2.1
Determine if each of the following molecules are chiral or achiral. For chiral molecules indicate any chiral carbons.

- Answer
-
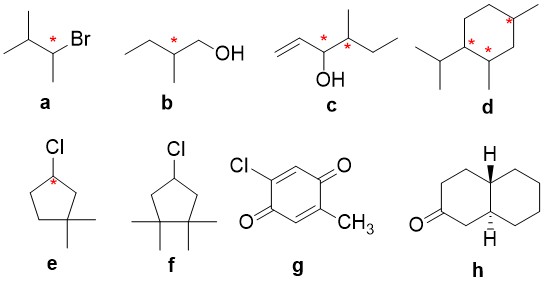
Explanation
Structures F and G are achiral. The former has a plane of symmetry passing through the chlorine atom and bisecting the opposite carbon-carbon bond. The similar structure of compound E does not have such a symmetry plane, and the carbon bonded to the chlorine is a chiral center (the two ring segments connecting this carbon are not identical). Structure G is essentially flat. All the carbons except that of the methyl group are sp2 hybridized, and therefore trigonal-planar in configuration. Compounds C, D & H have more than one chiral center, and are also chiral.
Example 5.2.2
Label the molecules below as chiral or achiral, and locate all stereocenters.
- Answer

Exercise 5.2.2
1) For the following compounds, star (*) each chiral center, if any.
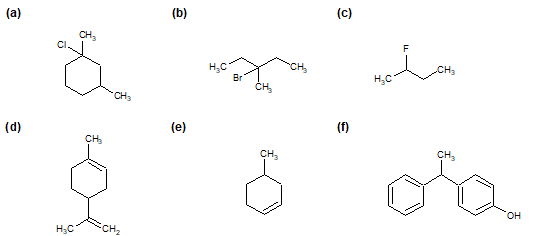
2) Explain why the following compound is chiral.

3) Determine which of the following objects is chiral.
a) A Glove.
b) A nail.
c) A pair of sunglasses.
d) The written word "Chiral".
4) Place an "*" by all of the chrial carbons in the following molecules.
a)
Erythrose, a four carbon sugar.

b) Isoflurane, an anestetic. Bright green = Chlorine, Pale green = Fluorine.
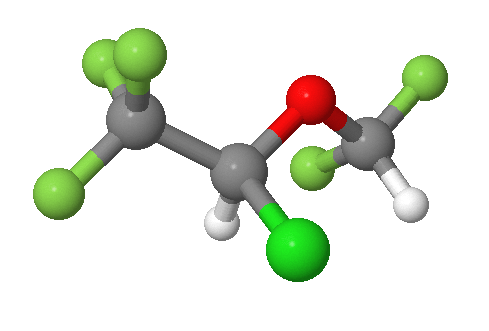
- Answer
-
1)
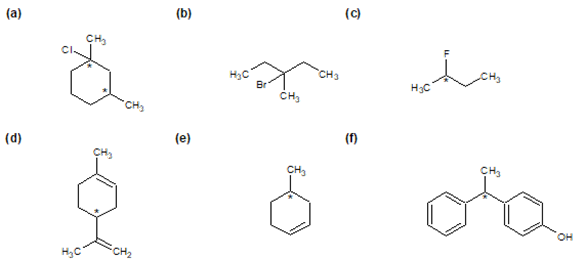
2) Though the molecule does not contain a chiral carbon, it is chiral as it is non-superimposable on its mirror image due to its twisted nature (the twist comes from the structure of the double bonds needing to be at 90° angles to each other, preventing the molecule from being planar).
3)
a) Just as hands are chiral a glove must also be chiral.
b) A nail has a plane of symmetry which goes down the middle making it a achiral.
c) A pair of sunglasses has a plane of symmetry which goes through the nose making it achiral.
d) Most written words are chiral. Look one in a mirror to confirm this.
4
a)

b)

Exercise 5.2.3
Circle all of the carbon stereocenters in the molecules below.

- Answer
-

Exercise 5.2.4
Circle all of the carbon stereocenters in the molecules below.

- Answer
-

Here are some more examples of chiral molecules that exist as pairs of enantiomers. In each of these examples, there is a single stereocenter, indicated with an arrow. (Many molecules have more than one stereocenter, but we will get to that that a little later!)
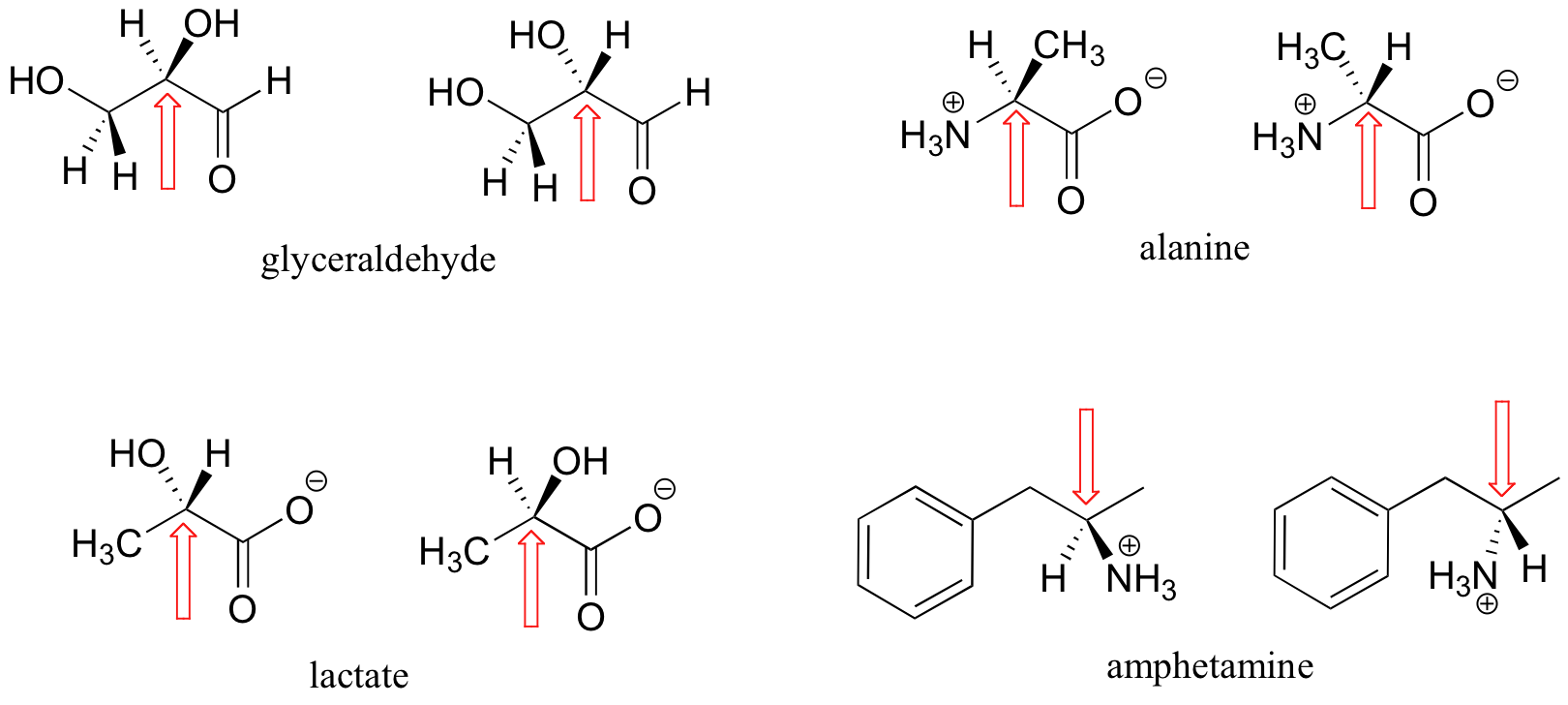
Here are some examples of molecules that are achiral (not chiral). Notice that none of these molecules has a stereocenter.
It is difficult to illustrate on the two dimensional page, but you will see if you build models of these achiral molecules that, in each case, there is at least one plane of symmetry, where one side of the plane is the mirror image of the other. Chirality is tied conceptually to the idea of asymmetry, and any molecule that has a plane of symmetry cannot be chiral. When looking for a plane of symmetry, however, we must consider all possible conformations that a molecule could adopt. Even a very simple molecule like ethane, for example, is asymmetric in many of its countless potential conformations – but it has obvious symmetry in both the eclipsed and staggered conformations, and for this reason it is achiral.
Looking for planes of symmetry in a molecule is useful, but often difficult in practice. In most cases, the easiest way to decide whether a molecule is chiral or achiral is to look for one or more stereocenters - with a few rare exceptions, the general rule is that molecules with at least one stereocenter are chiral, and molecules with no stereocenters are achiral. Carbon stereocenters are also referred to quite frequently as chiral carbons.
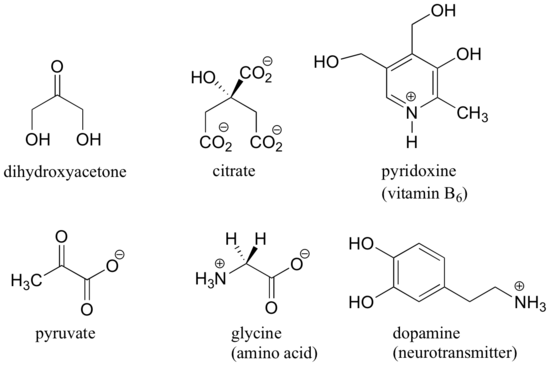
When evaluating a molecule for chirality, it is important to recognize that the question of whether or not the dashed/solid wedge drawing convention is used is irrelevant. Chiral molecules are sometimes drawn without using wedges (although obviously this means that stereochemical information is being omitted). Conversely, wedges may be used on carbons that are not stereocenters – look, for example, at the drawings of glycine and citrate in the figure above. Just because you see dashed and solid wedges in a structure, do not automatically assume that you are looking at a stereocenter.
Other elements in addition to carbon can be stereocenters. The phosphorus center of phosphate ion and organic phosphate esters, for example, is tetrahedral, and thus is potentially a stereocenter.

In order to investigate phosphate transfer reactions in living systems, especially involving ATP, researchers have incorporated sulfur and/or 17O and 18O isotopes of oxygen (the ‘normal’ isotope is 16O) to create chiral phosphate groups. Phosphate triesters are chiral if the three substituent groups are different.
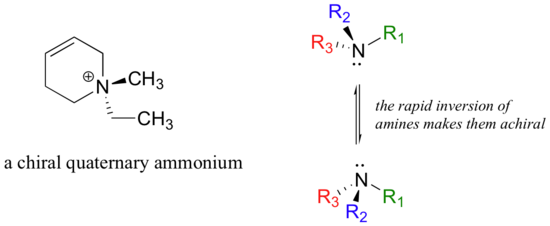
Asymmetric quaternary ammonium groups are also chiral. Amines, however, are not chiral, because they rapidly invert, or turn ‘inside out’, at room temperature.
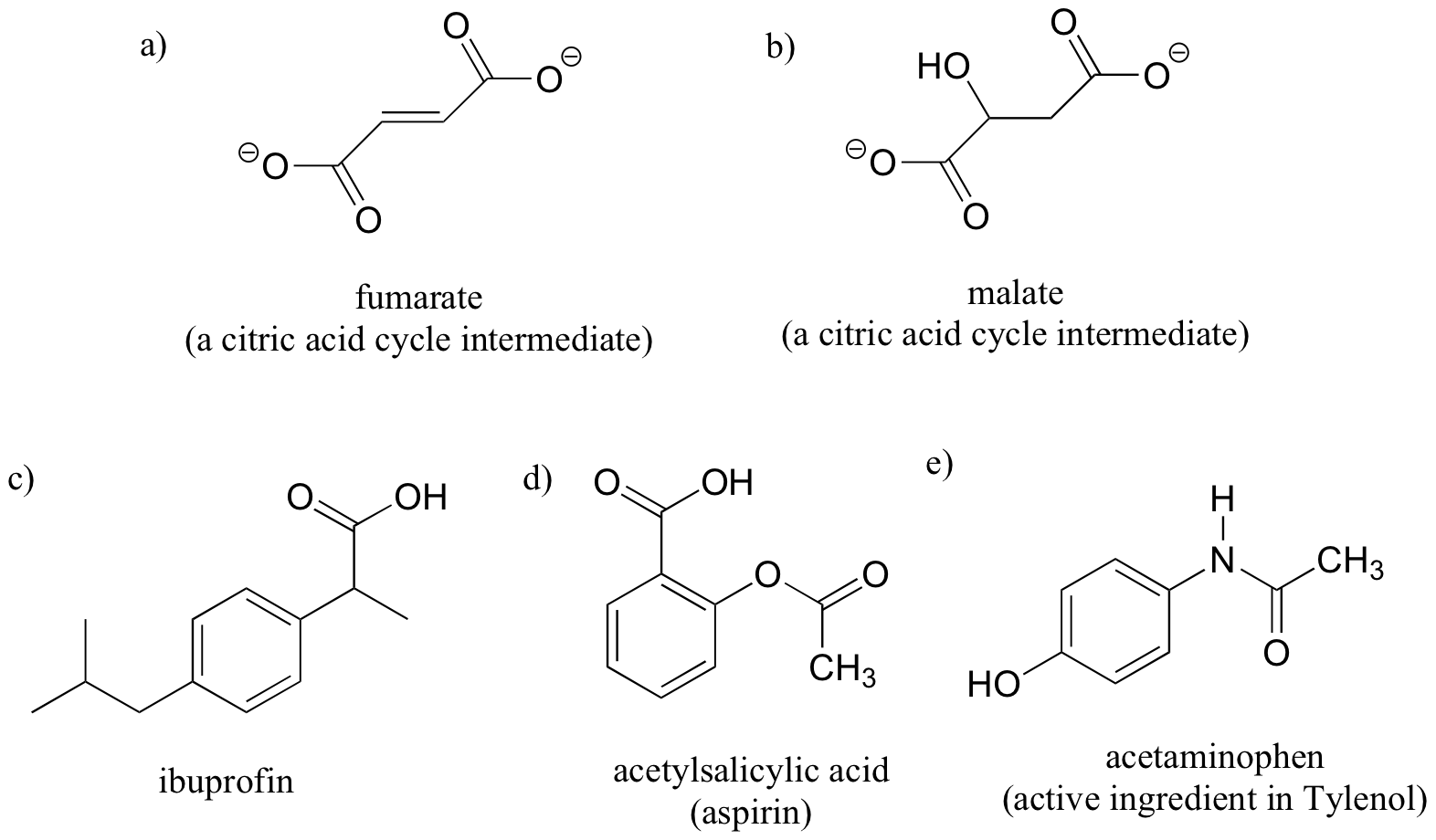
Exercise 5.2.5
Label the molecules below as chiral or achiral, and circle all stereocenters.
a) fumarate (a citric acid cycle intermediate)

b) malate (a citric acid cycle intermediate)

b) malate (a citric acid cycle intermediate)

- Answer
-
a) achiral (no stereocenters)

b) chiral

c) chiral

Exercise 5.2.6
Label the molecules below as chiral or achiral, and circle all stereocenters.
a) acetylsalicylic acid (aspirin)

b) acetaminophen (active ingredient in Tylenol)

c) thalidomide (drug that caused birth defects in pregnant mothers in the 1960’s)

- Answer
-
a) achiral (no stereocenters)

b) achiral (no stereocenters)

c) chiral

Exercise 5.2.7
Draw both enantiomers of the following chiral amino acids.
a) Cysteine 
b) Proline 
- Answer

-
-
-
Exercise 5.2.8
Draw both enantiomers of the following compounds from the given names.
a) 2-bromobutane
b) 2,3-dimethyl-3-pentanol
- Answer

-
Exercise 5.2.9
Which of the following body parts are chiral?
a) Hands b) Eyes c) Feet d) Ears
- Answer
-
a) Hands- chiral since the mirror images cannot be superimposed (think of the example in the beginning of the section)
b) Eyes- achiral since mirror images that are superimposable
c) Feet- chiral since the mirror images cannot be superimposed (Does your right foot fit in your left shoe?)
d) Ears- chiral since the mirror images cannot be superimposed
Exercise 5.2.10
Circle the chiral centers in the following compounds.

- Answer

-
Exercise 5.2.11
Identify the chiral centers in the following compounds.

- Answer

-














