Homework 29
- Page ID
- 28944
1.41
Classify Each statement as an Observation,Theory or Law.
- All matter that exists is made up of Submicroscopic particles known as atoms.
- If an iron rod is placed in a closed container and it rusts, the mass of the container and its contents do not change.
- Matter is neither created nor destroyed
- When a candle is burned, heat is released
Strategy:
You need to understand what an Observation, Theory or law is before you classify it in the question.
1. Observation: An outcome of events that is being viewed
2. Theory: Preserved explanation for observations and laws
3. Law: Summarizes past observations and predicts future ones
-Now that we have defined them, we need to apply it to the question (ANSWERS IN BOLD)
- This statement is proposing all matter is made up of atoms so it would become a theory because it is an idea that studies propose is true.
- This statement is saying, without matter being added to the container, As you See it rust, the mass doesn't change so this is assumed by Observation.
- This statement is called the Law of conservation of mass, so because this is true, the statement is a Law.
- If you are looking at a candle and see the flame, then you should understand that heat is being released. You tell this from Observation.
6.49
Write The Lewis Structure for each atom or molecule
- \(H_2O\)
- \(SF_6\)
- \(SO_2\)
- \(S^{2-}\)
Strategy
- Add up the electrons in each molecule so you can pair it correctly
- Find the central atom (least electronegative)
- Fill up the bonded atoms with electrons
- Fill up peripheral with left over atoms
- H atoms can only have electron and can only be shared with 1 other atom per Hydrogen
- For each (-) anion you add an electron and for each (+) cation you take away an electron
* 1st and 2nd Row Octet rules apply( 8 electrons to be happy) after 2 row Octet rule does not apply.
Solutions:
A). H20
- Count the electrons in each atom.
2 Hydrogen atoms = 2 electrons + 1 Oxygen atom 6 electrons Total: 8 electrons
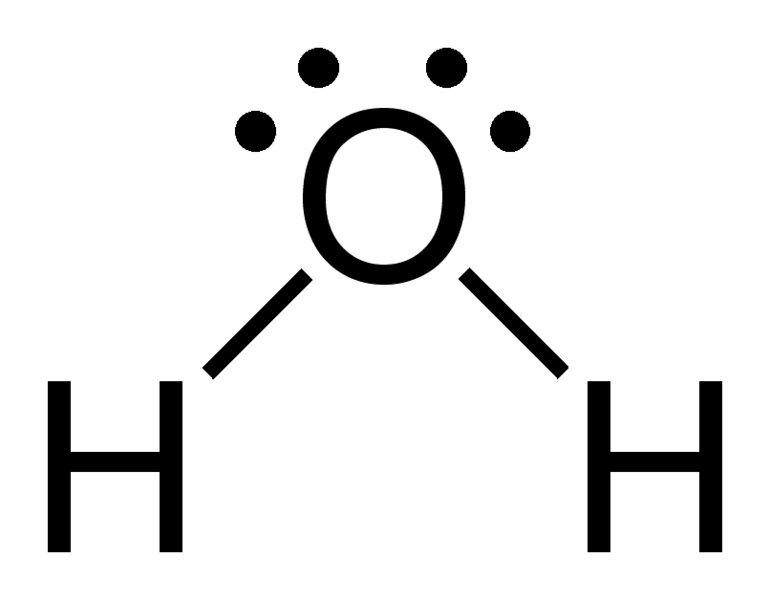 If you count all the electrons around each atoms, youll see there all filled up to be happy
If you count all the electrons around each atoms, youll see there all filled up to be happy
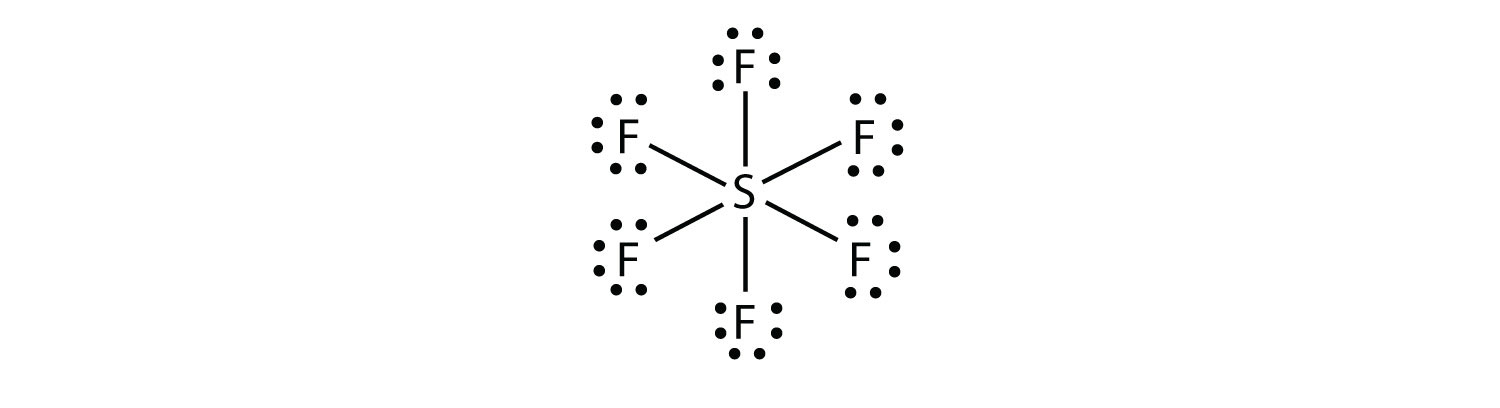
B).SF6
-Count the electrons in each atoms
1 Sulfur Atom= 6 electrons + 6 Fluoride Atoms= 42 electrons Total= 48 electrons
-Find the central atom and fill up each bonding atom first then peripheral sites.
- Central atom is sulfur (Least electronegative)
C). SO2
- Count electrons
1 Sulfur atom : 6 electrons + 2 Oxygen atoms : 12 electrons Total:
central atom : Sulfur
-Because the central atom would not be happy while filling up the atoms,in order to do so you have to make double bonds to fulfill the octet. Once you do that you should come up with these 3 options called resonance structures.
D.) S2-
- Sulfur originally has 6 electrons so now that there is a minus two sign above it, you then add another 2 electrons.. 6electrons + (2-) = 8 electrons in the sulfer atom.
- Now fill the orbitals with electrons untill empty.

