Homework 2
- Page ID
- 28859
1.55, 3.57
1.55
Problem:
According to Dalton's Atomic Theory, what are compounds made up of?
How do I approach this?
- Find the summary of Dalton's Atomic Theory
- Read through it
- Locate where it talks about what compounds are made of
- Write your answer
Solution:
Compounds are made up of two or more different atoms.
3.57
Problem:
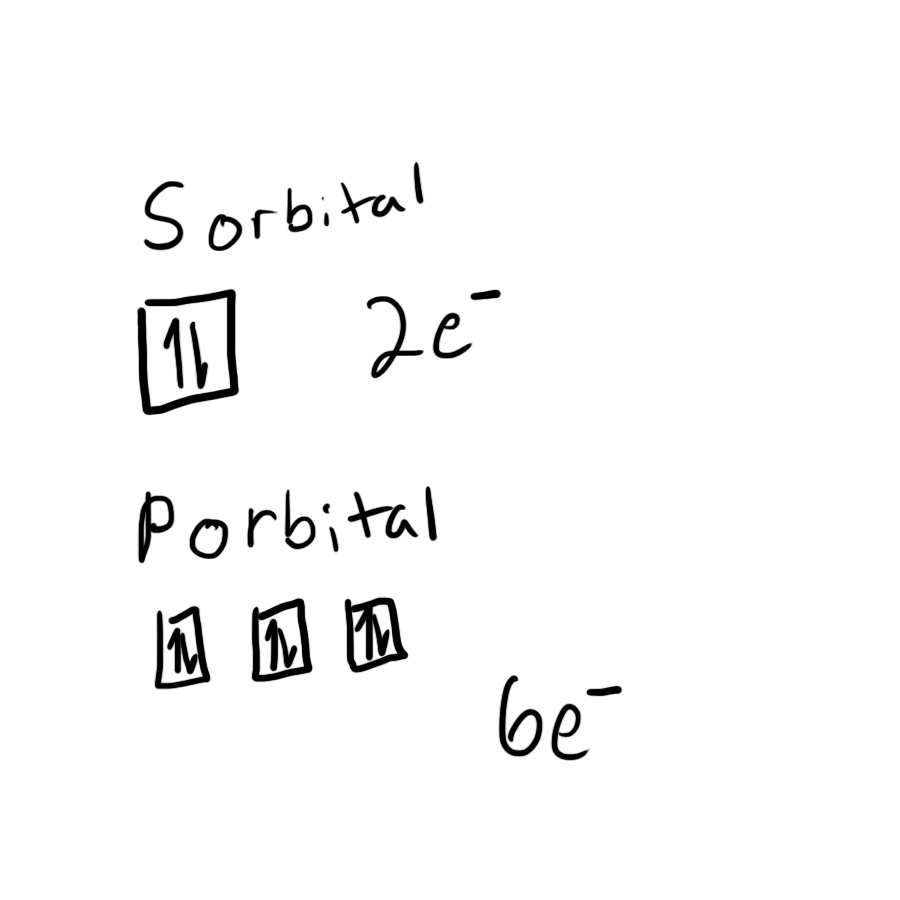
How many electrons are in a s orbital verses a p orbital?
How do I approach this?
Notation to know:
e- is the notation for an electron
Orbital- the energy state in an atom where an electron will be found
1. Figure out where an s orbital and p orbital would be located
2. Determine which level each one is
3. Use which level they are to determine how many boxes electrons can occupy
4. Realize that two opposite spinning electrons can occupy one box
5. Using the number of boxes and the knowledge that two electrons can occupy a box, multiply the number of boxes by the two orbitals
6. Write your answer
Solution:
The s orbital has one box that could have electrons in it since s is the lowest orbital. p is the next level up, which has three boxes electrons can occupy. Each box has the capacity for two electrons to occupy the space. Since s has one box, and p has three boxes, the number of electrons that could possibly be in an s orbital is two, while a p orbital has 6.