1.5: Slater's Rules
- Page ID
- 226579
Learning Objective
- To quantify the shielding effect experienced by atomic electrons.
We have previously described the concepts of electron shielding, orbital penetration and effective nuclear charge, but we did so in a qualitative manner. In this section, we explore one model for quantitatively estimating the impact of electron shielding, and then use that to calculate the effective nuclear charge experienced by an electron in an atom. The model we will use is known as Slater's Rules (J.C. Slater, Phys Rev 1930, 36, 57).
Slater's Rules
The general principle behind Slater's Rule is that the actual charge felt by an electron is equal to what you'd expect the charge to be from a certain number of protons, but minus a certain amount of charge from other electrons. Slater's rules allow you to estimate the effective nuclear charge \(Z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each orbital "shell" (e.g., to compare the effective nuclear charge and shielding 3d and 4s in transition metals). Slater's rules are fairly simple and produce fairly accurate predictions of things like the electron configurations and ionization energies.
Slater's Rules
- Step 1: Write the electron configuration of the atom in the following form:
(1s) (2s, 2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f) (5s, 5p) . . .
- Step 2: Identify the electron of interest, and ignore all electrons in higher groups (to the right in the list from Step 1). These do not shield electrons in lower groups
- Step 3: Slater's Rules is now broken into two cases:
- the shielding experienced by an s- or p- electron,
- electrons within same group shield 0.35, except the 1s which shield 0.30
- electrons within the n-1 group shield 0.85
- electrons within the n-2 or lower groups shield 1.00
- the shielding experienced by nd or nf valence electrons
- electrons within same group shield 0.35
- electrons within the lower groups shield 1.00
- the shielding experienced by an s- or p- electron,
These rules are summarized in Figure \(\PageIndex{1}\) and Table \(\PageIndex{1}\).
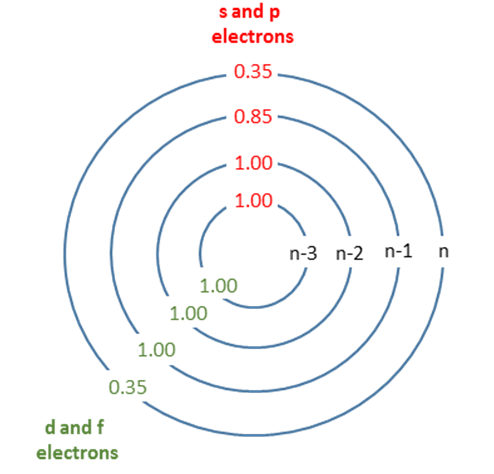
Shielding happens when electrons in lower valence shells (or the same valence shell) provide a repulsive force to valence electrons, thereby "negating" some of the attractive force from the positive nucleus. Electrons really close to the atom (n-2 or lower) pretty much just look like protons, so they completely negate. As electrons get closer to the electron of interest, some more complex interactions happen that reduce this shielding.
| Group | Other electrons in the same group | Electrons in group(s) with principal quantum number n and azimuthal quantum number < l | Electrons in group(s) with principal quantum number n-1 | Electrons in all group(s) with principal quantum number < n-1 |
|---|---|---|---|---|
| [1s] | 0.30 | - | - | - |
| [ns,np] | 0.35 | - | 0.85 | 1 |
| [nd] or [nf] | 0.35 | 1 | 1 | 1 |
The shielding numbers in Table \(\PageIndex{1}\) were derived semi-empirically (i.e., derived from experiments) as opposed to theoretical calculations. This is because quantum mechanics makes calculating shielding effects quite difficult, which is outside the scope of this Module.
Calculating S
Sum together the contributions as described in the appropriate rule above to obtain an estimate of the shielding constant, \(S\), which is found by totaling the screening by all electrons except the one in question.
\[ S = \sum n_i S_i \label{2.6.0}\]
where
- \(n_i\) is the number of electrons in a specific shell and subshell and
- \(S_i\) is the shielding of the electrons subject to Slater's rules (Table \(\PageIndex{1}\))
Example \(\PageIndex{1}\): The Shielding of 3p Electrons of Nitrogen Atoms
What is the shielding constant experienced by a 2p electron in the nitrogen atom?
Given: Nitrogen (N)
Asked for: \(S\), the shielding constant, for a 2p electron (Equation \ref{2.6.0})
Strategy:
- Determine the electron configuration of nitrogen, then write it in the appropriate form.
- Use the appropriate Slater Rule to calculate the shielding constant for the electron.
Solution A N: 1s2 2s2 2p3
N: (1s2)(2s2,2p3)
Solution B
\[S[2p] = \underbrace{0.85(2)}_{\text{the 1s electrons}} + \underbrace{0.35(4)}_{\text{the 2s and 2p electrons}} = 3.10\nonumber\]
As Table \(\PageIndex{1}\) indicates,
- the 1s electrons shield the other 2p electron to 0.85 "charges".
- the 2s and 2p electrons shield the other 2p electron equally at 0.35 "charges".
Exercise \(\PageIndex{1}\): The Shielding of valence p Electrons of Bromine Atoms
What is the shielding constant experienced by a valence p-electron in the bromine atom?
- Answer
- \[S = 2+8+8 \times 0.85 + 10 + 4 \times 0.35 = 28.20 \nonumber \]
Example \(\PageIndex{2}\): The Shielding of 3d Electrons of Bromine Atoms
What is the shielding constant experienced by a 3d electron in the bromine atom?
Given: Bromine (Br)
Asked for: S, the shielding constant, for a 3d electron
Strategy:
- Determine the electron configuration of bromine, then write it in the appropriate form.
- Use the appropriate Slater Rule to calculate the shielding constant for the electron.
Solution A Br: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Br: (1s2)(2s2,2p6)(3s2,3p6)(3d10)(4s2,4p5)
Ignore the group to the right of the 3d electrons. These do not contribute to the shielding constant.
Solution B S[3d] = 1.00(18) + 0.35(9) = 21.15
Exercise \(\PageIndex{2}\): The Shielding of 3d Electrons of Copper Atoms
What is the shielding constant experienced by a valence d-electron in the copper atom?
- Answer
- S = 21.15
Calculating Zeff
One set of estimates for the effective nuclear charge (\(Z_{eff}\)) was presented in Figure 2.5.1. Previously, we described \(Z_{eff}\) as being less than the actual nuclear charge (\(Z\)) because of the repulsive interaction between core and valence electrons. We can quantitatively represent this difference between \(Z\) and \(Z_{eff}\) as follows:
\[ S=Z-Z_{eff} \label{2.6.1} \]
Rearranging this formula to solve for \(Z_{eff}\) we obtain:
\[ Z_{eff}=Z-S \label{2.6.2} \]
We can then substitute the shielding constant obtained using Equation \(\ref{2.6.2}\) to calculate an estimate of \(Z_{eff}\) for the corresponding atomic electron.
Example \(\PageIndex{3}\): The Effective Charge of p Electrons of Boron Atoms
What is the effective nuclear charge experienced by a valence p- electron in boron?
Given: Boron (B)
Asked for: \(Z_{eff}\) for a valence p- electron
Strategy:
- Determine the electron configuration of boron and identify the electron of interest.
- Use the appropriate Slater Rule to calculate the shielding constant for the electron.
- Use the Periodic Table to determine the actual nuclear charge for boron.
- Determine the effective nuclear constant.
Solution:
A B: 1s2 2s2 2p1 . The valence p- electron in boron resides in the 2p subshell.
B: (1s2)(2s2,2p1)
B S[2p] = 1.00(0) + 0.85(2) + 0.35(2) = 2.40
C Z = 5
D Using Equation \ref{2.6.2}, \(Z_{eff} = 2.60\)
Exercise \(\PageIndex{3}\)
What is the effective nuclear charge experienced by a valence d-electron in copper?
- Answer
- \(Z_{eff} = 7.85 \)
Summary
Slater's Rules can be used as a model of shielding. This permits us to quantify both the amount of shielding experienced by an electron and the resulting effective nuclear charge. Others performed better optimizations of \(Z_{eff}\) using variational Hartree-Fock methods. For example, Clementi and Raimondi published "Atomic Screening Constants from SCF Functions." J Chem Phys (1963) 38, 2686–2689.
References
- James L. Reed, "The Genius of Slater's Rules" , J. Chem. Educ., 1999, 76 (6), p 802
- David Tudela, "Slater's rules and electron configurations", J. Chem. Educ., 1993, 70 (11), p 956
- Kimberley A. Waldron, Erin M. Fehringer, Amy E. Streeb, Jennifer E. Trosky and Joshua J. Pearson, "Screening Percentages Based on Slater Effective Nuclear Charge as a Versatile Tool for Teaching Periodic Trends", J. Chem. Educ., 2001, 78 (5), p 635
Contributors
Brett McCollum (Mount Royal University)

