2.5: A new state energy - enthalpy
- Page ID
- 322792
The knowledge that more heat has to transfer into a gas to change its temperature under constant pressure than at constant volume also begs the introduction of a new quantity. We have already argued that the change in internal energy of a system is exactly the heat transferred between that system and its surroundings when that system is held at constant volume:
Let’s define a new state energy - we will call it the enthalpy of a system, and we will give that energy the symbol H - to deal with the realities of the behavior of a system at constant pressure, rather than constant volume. We will define this quantity in this way:
If we talk about how this state function changes over time, and we specify that this change will be under conditions of constant pressure, then we can write that change as
But P ΔV is nothing more or less than the negative of the work done on the system, which simplifies the expression to the point where we can apply the first law of thermodynamics:
Therefore, while the change of internal energy is the heat transferred to that system at constant volume, we have defined this new quantity such that the change of enthalpy of a system is the heat transferred to that system at constant pressure. This is the connection between heat and enthalpy that has been talked about loosely ever since you took general chemistry. Now that we have defined and declared this definition formally, it is necessary that we keep this definition of enthalpy change specific; we will use it repeatedly going forward.
The introduction of the concept of enthalpy is where we move into the specific discipline of chemical thermodynamics from the general study of thermodynamics. Enthalpy is a concept that doesn't get addressed, or is only addressed in passing, in most general treatments of the transfer of heat, but the enthalpy concept is powerful in chemistry because it takes a transfer of heat under specific conditions - the most common laboratory conditions, constant pressure - and identifies it as a state energy. This makes the enthalpy concept supremely useful to the chemist, because it allows for the prediction of heat transfer from complex chemical reactions from other, more well-characterized chemical reactions.
This is the conceptual description of the principle of chemistry known as Hess' Law - if a series of chemical processes starts with materials A and ends with materials B, the enthalpy change for that series of chemical processes is equivalent to the enthalpy change corresponding with the direct reaction of materials A to materials B. Let's give a specific example of this, the example of the famous thermite reaction:
This reaction is the sum of two other reactions, the endothermic deformation of iron(III) oxide to its constituent elements in their standard states, and the highly exothermic formation of aluminum oxide from its constituent elements in their standard states:
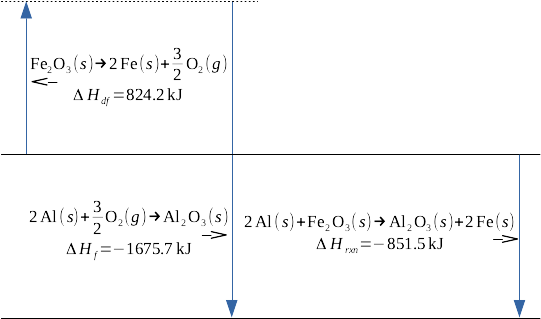
Formation reactions are well-characterized for a wide variety of products; any standard table of thermodynamic data is a good starting point for drawing this data. I'm using deformation, or df, to represent the decomposition of a reactant to its constituent elements in standard states; it's the opposite of a formation reaction; the enthalpy of deformation is the negative of the enthalpy of formation.
Determining an enthalpy of reaction for a process like the thermite reaction, then, involves taking the arithmetic sum of the enthalpies of deformation of the reactants and the enthalpies of formation of the products. This statement of Hess' Law is expressed in many general chemistry textbooks as simply taking the the sum of the enthalpies of formation of products, and subtracting from that the sum of the enthalpies of formation of the reactants:
The degree-symbol applied to the enthalpy symbol ΔH° represents the change in enthalpy at standard ambient temperature and pressure (SATP; 1 atmosphere and 298 K, or 25°C). This is implied frequently in general chemistry work, but we'll need to be explicit about the distinction between SATP and non-standard conditions later.
Take another reaction as an example, the combustion of propane gas in the presence of oxygen to products of carbon dioxide and water vapor:
This reaction has multiple moles of reactants and products, in addition to a quantity of oxygen in its standard state (and the enthalpy of formation of any element in its standard state is zero). The enthalpies of formation of the compound product as well as the reactants can be stated as molar quantites:
The application of the equation form of Hess' Law to this reaction is therefore the sum of the enthalpies of formation of carbon dioxide and water vapor, each multiplied by their stoichiometric coefficients, subtracting the enthalpy of formation of propane:
The value of -2043.9 kJ mol-1 has to be interpreted carefully. The reactant with the stoichiometric coefficient of 1 is the propane itself, C3H8. Therefore the standard enthalpy of reaction for the combustion of propane is -2043.9 kJ of energy released per mole of propane combusted. This brings us to an inconsistency in our notation system: frequently enthalpies of formation and enthalpies of reaction are provided as molar quantities, and in the name of consistency should be presented with a bar over it. This is rare to see in textbooks, however, and it's frequently simply assumed that is a molar quantity without any further notation provided. We'll make more effort to be consistent in the future, but Hess' Law is one of the places where it's most commonly assumed that enthalpies are molar quantites.
Hess' Law is one of the primary applications of the enthalpy concept, and the application of the enthalpy concept most clearly connected to chemistry. But there are other applications of the enthalpy concept. At constant pressure, any heat transfer represented by q can be written as an enthalpy change ΔH. There are also values of enthalpy associated with phase changes, the transformation of matter from one phase to another. The enthalpy of fusion for water's transformation between liquid and solid states is ΔHfus = 6.01 kJ mol-1 = 333.6 J g-1; one mole of ice absorbs 6010 J of energy as it melts to water, and one mole of water releases 6010 J of energy as it freezes to ice. Likewise, the enthalpy of vaporization for water's transformation between gaseous and liquid states is ΔHvap = 40.67 kJ mol-1 = 2.258 kJ g-1.

