Although four- and six-coordinate geometries are probably the most commonly observed in inorganic chemistry, a range of other shapes have also been reported.1 The other most common coordination number is five. Geometries for five-coordination include both trigonal bipyramidal and square pyramidal. Both are common, and distorted geometries in between these two limiting cases are even more commonly observed. The fractional parameter, τ (tau), is usually reported with structural analyses of five-coordinate compounds to convey where the structure falls on this continuum. A value of τ = 1 corresponds to perfect trigonal bipyramidal and a value of τ = 0 corresponds to perfect square pyramidal. Most reported complexes have values solidly in between these extremes. However, there are some ligands, such as porphyrins, in which a rigid ring of four basal donors enforces square pyramidal geometry more closely.
Lower coordination numbers are less common but are reported in certain cases. Two-coordinate compounds are sometimes observed in complexes of the coinage metals. They include a range of ligands, including σ donors in [Ag(NH3)2]+ ion, π donors in [CuCl2]- ion and π acceptors in [Au(CN)2]- ion, for example. Three coordinate complexes have been reported from across the periodic table, especially with sterically demanding ligands such as Bradley and Chisholm's [(Me3Si)2N]3M (Sc, Ti, V, Cr, Fe) or Wolczanski's (t-Bu3SiO)3Ta.2,3
Coordination numbers higher than six are sometimes observed. These cases often involve early transition metals in high oxidation states that are less electronically saturated. Examples are also seen in the lanthanides and actinides owing to their large size. There is even a reported sixteen-coordinate complex, although it is of an alkali metal ion and not a transition metal ion.
References
1. Gispert, J. R. Coordination Chemistry, Wiley-VCH: Weinheim, Germany, 2008, pp. 59-80.
2. Bradley, D. C.; Copperthwaite, R. G.; Extine, M. W.; Reichert, W. W.; Chisholm, M. H. (1978). "Transition Metal Complexes of Bis(Trimethyl-silyl)Amine (1,1,1,3,3,3-Hexamethyldisilazane)" Inorganic Syntheses. 1978, 18. p. 112.
3. Neithamer, D. R.; LaPointe, R. E.; Wheeler, R. A.; Richeson, D. S.; Van Duyne, G. D.; Wolczanski, P. T. "Carbon monoxide cleavage by (silox)3Ta (silox = tert-Bu3SiO-): physical, theoretical, and mechanistic investigations", J. Am. Chem. Soc. 1989, 111, 25, 9056-9072.
4. Pollak, D.; Goddard, R.; Pörschke, K.-R. "Cs[H2NB2(C6F5)6] Featuring an Unequivocal 16-Coordinate Cation". J. Am. Chem. Soc. 2016, 138, 30, 9444-9451.
Problems
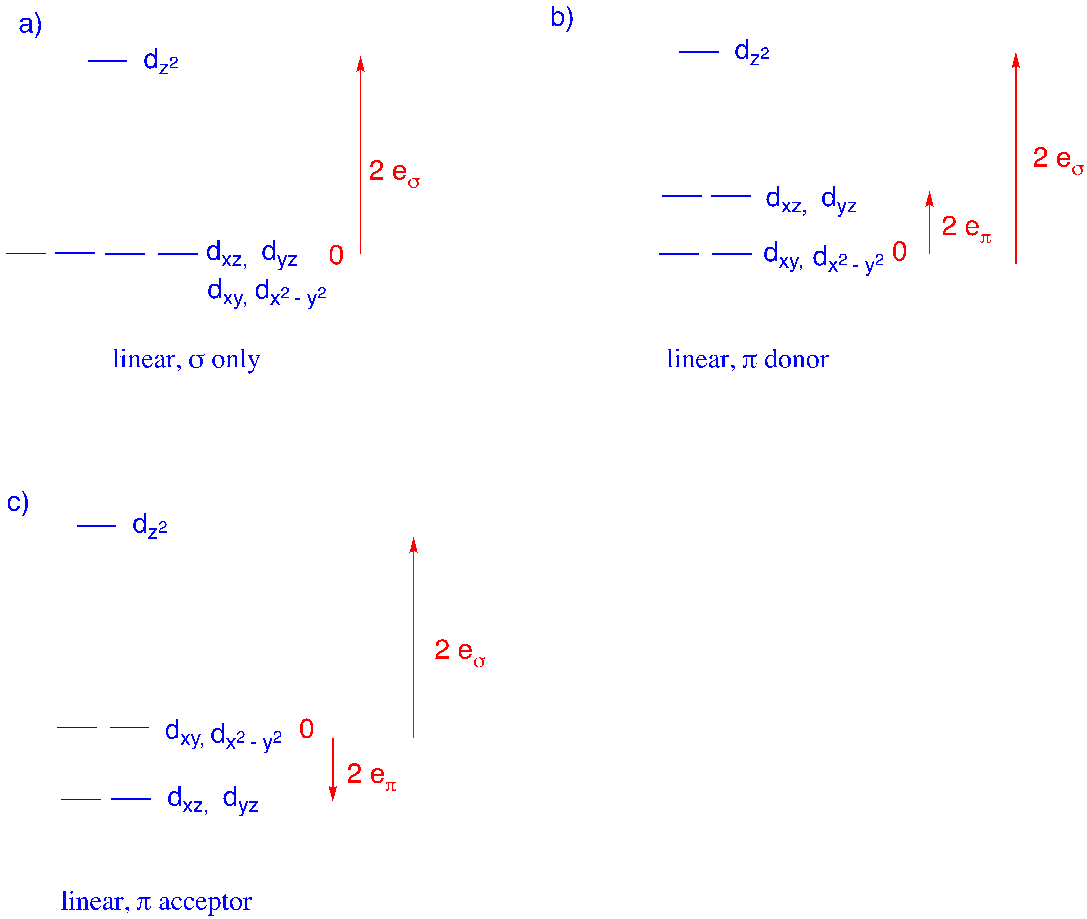
1. Use the angular overlap model to
i) calculate d orbital energy destabilization and
ii) construct d orbital splitting diagrams for linear (two-coordinate) geometry with the following ligand types.
a) sigma donor b) pi acceptor c) pi donor
2. Use the angular overlap model with sigma-only interactions to calculate d orbital energy destabilization in square pyramidal geometry.
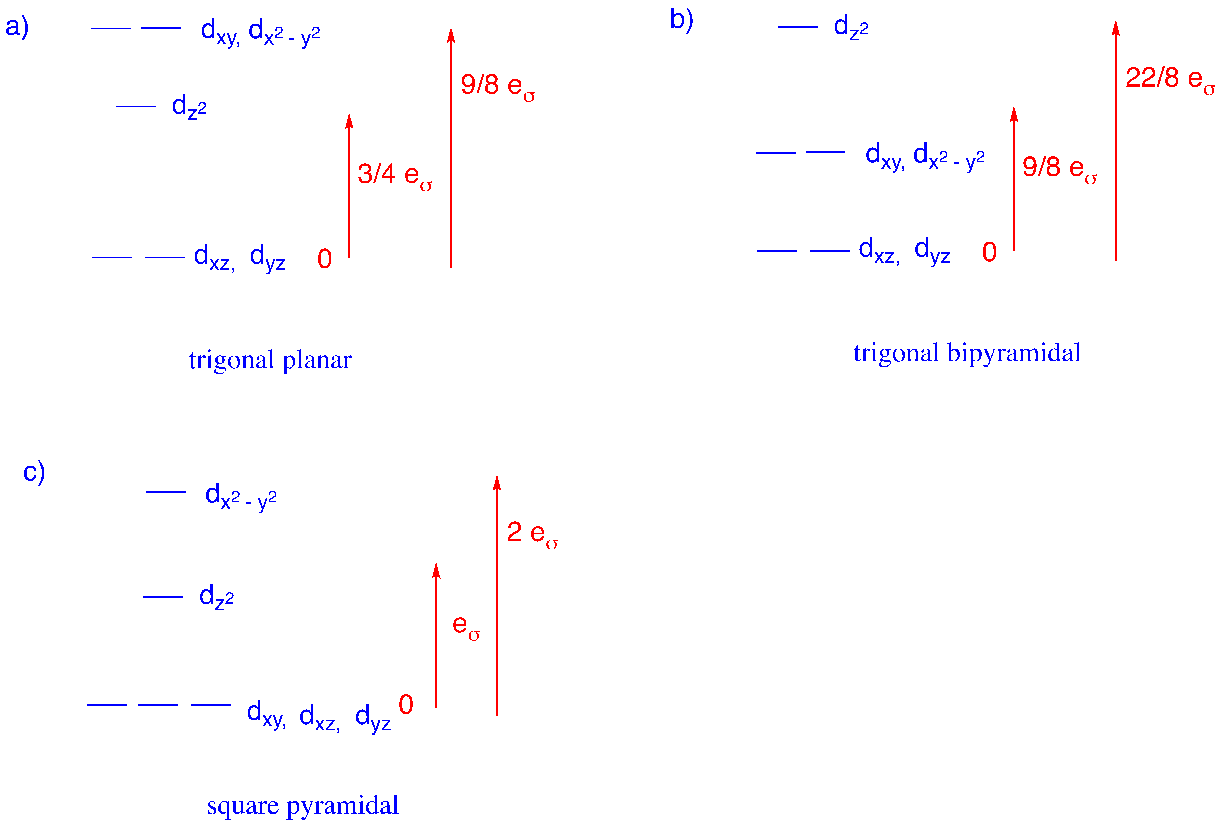
3. Use the angular overlap model with sigma-only interactions to construct d orbital splitting diagrams for the following geometries.
a) trigonal planar b) trigonal pyramidal c) square bipyramidal
Solutions
1. i) a) Positions 1, 6.
dz2: (1 + 1) eσ = 2 eσ
dx2-y2: (0 + 0) eσ = 0
dxy: (0 + 0) eσ = 0
dxz: 0
dyz: 0
b) Positions 1, 6.
dz2: 0
dx2-y2: - (0 + 0) eπ = 0
dxy: - (0 + 0 ) eπ = 0
dxz: - (1 + 1) eπ = -2 eπ
dyz: - (1 + 1) eπ = -2 eπ
c) Positions 1, 6.
dz2: 0
dx2-y2: (0 + 0) eπ = 0
dxy: (0 + 0 ) eπ = 0
dxz: (1 + 1) eπ = 2 eπ
dyz: (1 + 1) eπ = 2 eπ
ii)
2. Positions 1, 2, 3, 4, 5:
dz2: (1 + 1/4 + 1/4 + 1/4 + 1/4) eσ = 2eσ
dx2-y2: (0 + 3/4 + 3/4 + 3/4 + 3/4) eσ = 3eσ
dxy: 0
dxz: 0
dyz: 0
3.