Students sometimes want to be able to predict the coordination geometry that will be adopted by a particular combination of metal ion and ligands. In reality, there are enough variables involved in both the ligand and the metal that such predictions are not possible with any certainty.
Nevertheless, there are some broad trends that can be highlighted by looking at what the angular overlap model says about geometric preferences. One of the most common questions is whether a four coordinate complex will adopt a tetrahedral or square planar geometry. Probably the most important factor in this case is the steric bulk of the ligands. The ligand-metal-ligand bond angles in tetrahedral geometry are ideally 109 degrees, compared to 90 degrees in square planar geometry. As a result, ligands are spaced further apart in tetrahedral geometry than in square planar geometry, so bulkier ligands favor tetrahedral complexes.
On the other hand, the angular overlap model (as well as other approaches) suggests some advantages of square planar geometry. A comparison of eσ at a range of d electron configurations for both high spin and low spin cases is plotted below.
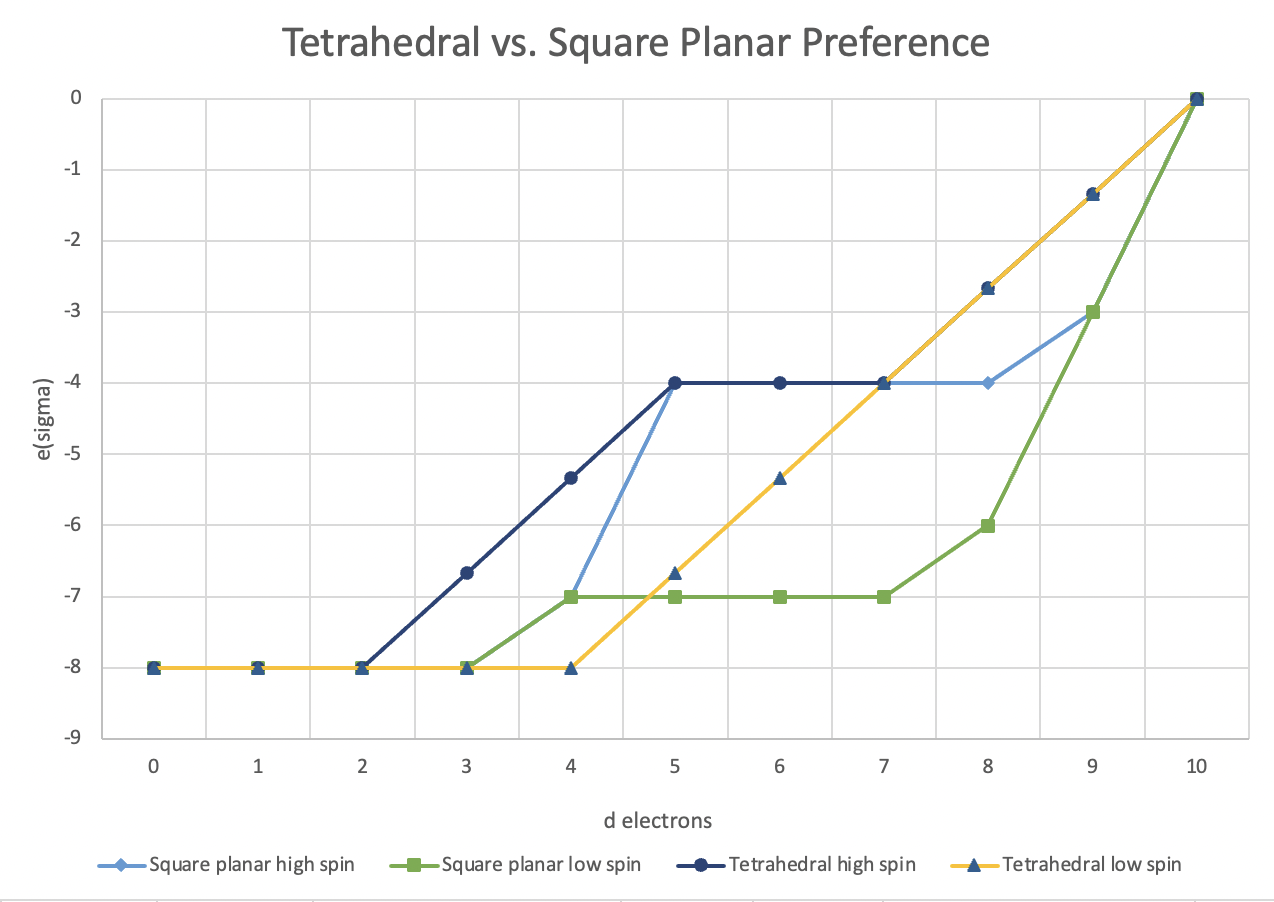
From the outset, we should note that low spin tetrahedral complexes are almost unheard of, so we should really compare square planar to the high spin tetrahedral case. In the comparison between high spin tetrahedral and high spin square planar geometry, square planar is favored only for d3, d4, d8 and d9 configuration. However, as we will see shortly, square planar complexes of d3 and d4 metals are not very common because metals with those electron configurations are more likely to adopt higher coordination geometries, such as five or six coordination. All of the other cases are more likely to adopt a tetrahedral geometry because if electronic stabilization factors are equal then steric factors (relief of ligand crowding) will prevail.
If we are comparing low spin square planar to high spin tetrahedral cases, there is an increased preference for the square planar geometry for all but the d1, d2 and d10 configurations. That suggests square planar geometry is usually favored unless there are steric factors that push the geometry to tetrahedral. Of course, the comparison we have used ignores eπ. Because the magnitude of eσ and eπ vary with different ligands and metals, we can't easily include both factors in one graph. However, we do know that π donors will make it more likely that we are dealing with a high spin square planar case, whereas π acceptors will lead to a low spin square planar case. These two cases have a dramatic effect on the d orbital splitting diagram. The angular overlap model suggests a dramatic reordering of the square planar d orbital splitting diagram in these cases.
Consequently, square planar geometry is expected to be much more common in cases with π acceptor ligands, whereas tetrahedral geometry should be more common in cases with π donor ligands.
It is worth noting that the first reported group of low spin tetrahedral complexes, the crystallographically characterized tetrakis(norbornyl)cobalt(IV), (nor)4Co, as well as the cationic and anionic (nor)4Co+ and (nor)4Co-, are d5, d4 and d6 complexes, respectively.1 AOM predicts very similar stabilization in low spin square planar and low spin tetrahedral geometries in these cases. However, the tetrahedral geometry is largely enforced by the bulky norbornyl ligands.
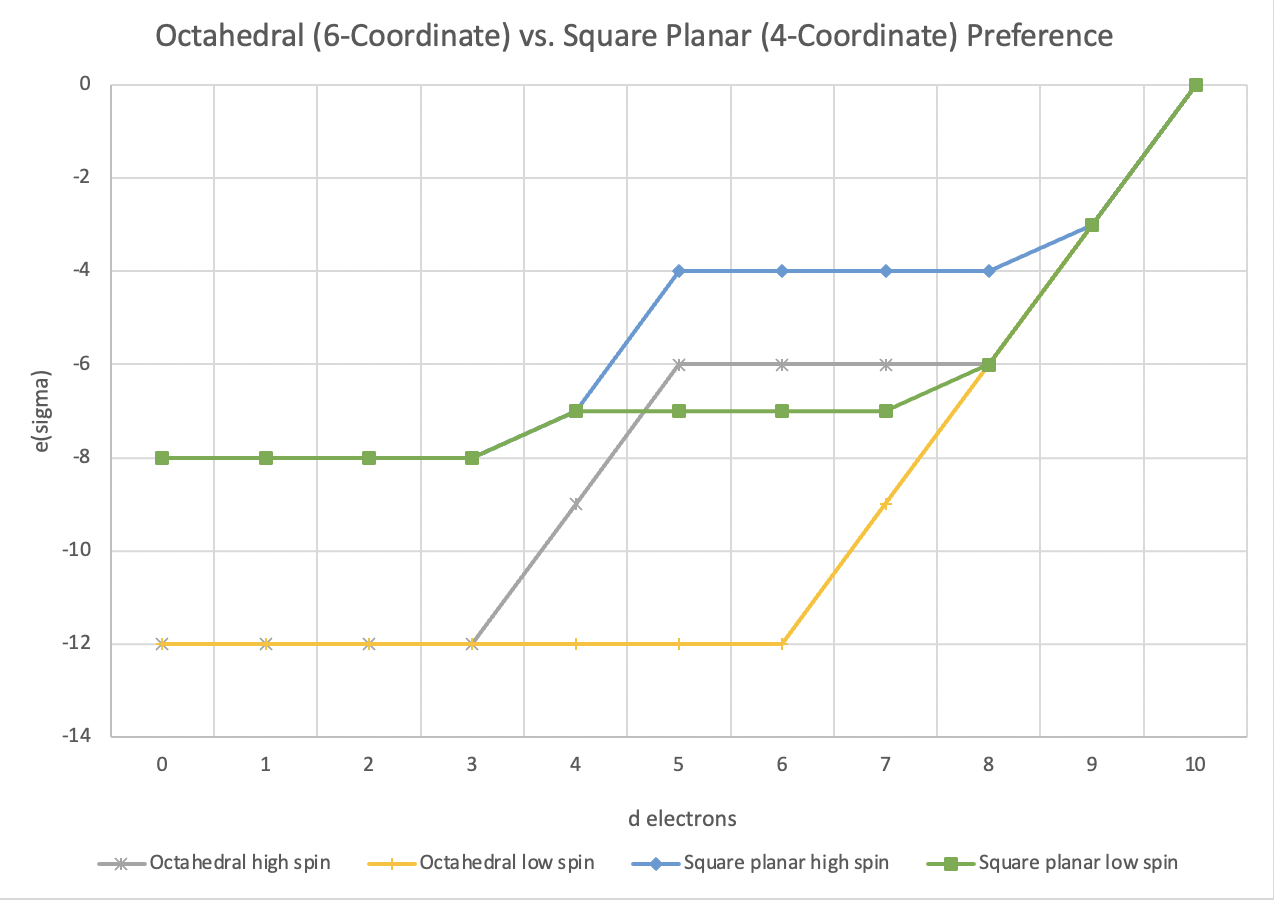
As mentioned above, there is a general preference for six coordination over four coordination, especially at lower d electron counts. A simple AOM comparison indicates octahedral geometry is favored for the low spin case between d0 and d7. Even high spin octahedral geometry is favored electronically over low spin square planar up to d4, with only a modest preference for square planar between d5 and d7. It isn't surprising that octahedral geometry is so common. In very simple terms, this preference comes from the energetically favored formation of six metal-ligand bonds rather than only four metal-ligand bonds.
Example \(\PageIndex{1}\)
Confirm the stabilization values from the angular overlap model for (a) high spin and (b) low spin tetrahedral geometry.
Solution
Stabilization (σ only) ΔE = 0(# e electrons) - 43(# t2 electrons) - 4(2 electrons per ligand) eσ
a) For high spin:
- d0: ΔE = 0(0) + 43(0) - 8 eσ = -8 eσ
- d1: ΔE = 0(1) + 43 (0) - 8 eσ = -8 eσ
- d2: ΔE = 0(2) + 43 (0) - 8 eσ = -8 eσ
- d3: ΔE = 0(2) + 43 (1) - 8 eσ = -623 eσ
- d4: ΔE = 0(2) - 43(2) - 8 eσ = -513 eσ
- d5: ΔE = 0(2) - 43 (3) - 8 eσ = -4 eσ
- d6: ΔE = 0(3) - 43 (3) - 8 eσ = -4 eσ
- d7: ΔE = 0(4) - 43 (3) - 8 eσ = -4 eσ
- d8: ΔE = 0(4) - 43 (4) - 8 eσ = -223 eσ
- d9: ΔE = 0(4) - 43 (5) - 8 eσ = -113 eσ
- d10: ΔE = 0(4) - 43 (6) - 8 eσ = 0 eσ
b) For low spin:
- d0: ΔE = 0(0) + 43 (0) - 8 eσ = -8 eσ
- d1: ΔE = 0(1) + 43 (0) - 8 eσ = -8 eσ
- d2: ΔE = 0(2) + 43 (0) - 8 eσ = -8 eσ
- d3: ΔE = 0(3) + 43 (0) - 8 eσ = -8 eσ
- d4: ΔE = 0(4) -43 (0) - 8 eσ = -8 eσ
- d5: ΔE = 0(4) - 43 (1) - 8 eσ = -623 eσ
- d6: ΔE = 0(4) - 43 (2) - 8 eσ = -513 eσ
- d7: ΔE = 0(4) - 43 (3) - 8 eσ = -4 eσ
- d8: ΔE = 0(4) - 43 (4) - 8 eσ = -223 eσ
- d9: ΔE = 0(4) - 43 (5) - 8 eσ = -113 eσ
- d10: ΔE = 0(4) - 43 (6) - 8 eσ = 0 eσ
Example \(\PageIndex{2}\)
Calculate the preference for square planar geometry for each d electron count in (a) the case of high spin square planar vs. high spin tetrahedral, and (b) the case of low spin square planar vs. high spin tetrahedral.
Solution
Preference = ΔΔE = ΔE(square planar) - ΔE(tetrahedral)
a) For high spin:
- d0: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d1: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d2: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d3: ΔΔE = - 8 - (-623 )eσ = -113eσ
- d4: ΔΔE = -7 - (-513) eσ = -123eσ
- d5: ΔΔE = -4 - (4) eσ = 0 eσ
- d6: ΔΔE = -4 - (4) eσ = 0 eσ
- d7: ΔΔE = -4 - (4) eσ = 0 eσ
- d8: ΔΔE = -4 - (223) eσ = -113 eσ
- d9: ΔE = -3 - (113) eσ = -123 eσ
- d10: ΔE = 0 - (0) eσ = 0 eσ
b) For low spin:
- d0: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d1: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d2: ΔΔE = - 8 -(- 8) eσ = 0 eσ
- d3: ΔΔE = - 8 - (-623 )eσ = -113eσ
- d4: ΔΔE = -7 - (-513) eσ = -123eσ
- d5: ΔΔE = -7 - (4) eσ = -3 eσ
- d6: ΔΔE = -7 - (4) eσ = -3 eσ
- d7: ΔΔE = -7 - (4) eσ = -3 eσ
- d8: ΔΔE = -6 - (223) eσ = -313 eσ
- d9: ΔE = -3 - (113) eσ = -123 eσ
- d10: ΔE = 0 - (0) eσ = 0 eσ
References
1. Byrne, E. K.; Theopold, K. H. "Synthesis, Characterization, and Electron-Transfer Reactivity of Norbornyl Complexes of Cobalt in Unusually High Oxidation States." J. Am. Chem. Soc. 1989, 111, 3887-3896.


