9.6: Coordination Frameworks
- Last updated
- Save as PDF
- Page ID
- 377701
Bridging ligands link metals in multicenter coordination complexes
Some ambidentate and multidentate ligands have the capacity to bridge two metal centers. For instance, the cyano ligands can bridge two metal centers by forming linear links of the type
\[\ce{M-CN-M'} \nonumber \]
Linkages like these can be used to bridge multiple metal centers in a cluster or network solid, the latter of which is sometimes called a coordination framework. When the cyano ligand is combined with octahedral metal centers possessing 90\(^{\circ}\) L-M-L bond angles, a cubic coordination network results. An example is the Prussian Blue coordination framework, which possesses the unit cell depicted in Figure \(\sf{\PageIndex{1A}}\).
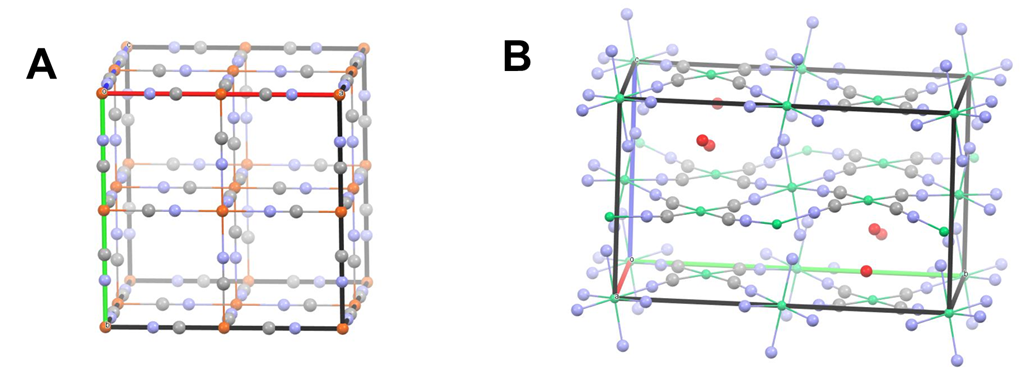
The cubic network shown in Figure \(\sf{\PageIndex{1A}}\) reflects the 3D structure of the octahedron, in which the M-L bonds point along the x, y, and z axes. If a square planar metal center is used, a 2D coordination network comprising interlinked squares is produced instead. An example is the Hofmann clathrate shown in Figure \(\sf{\PageIndex{1B}}\), which depicts a Hofmann clathrate in which square planar [Ni(CN)4]2- units are linked by trans-Ni(NH3)22+ units (which are octahedral but have a square plane of open coordination sites perpendicular to the NH3-Ni-NH3 axis).
The structures of coordination polyhedra and frameworks reflect ligand and metal coordination geometries
As illustrated by the Prussian Blue and Hofmann clathrate coordination networks shown in Figure \(\sf{\PageIndex{1}}\), the structures formed when a bridging ligand links multiple metal centers depends on the geometry of the centers, the ligand, and the metal-ligand bonds formed. Because the directionality of metal-ligand bonding in many types of complexes is well-characterized and predictable, it is possible to design metal complexes and linkers that can serve as building blocks for clusters and networks. In particular
- metal complexes with combinations of labile (substitutable) ligands in particular topologies. These insure that the linking ligand will be arranged around the metal center in those defined directions.
- Ligands that possess binding sites oriented in defined directions so that they link the metal centers together in particular topologies.
Examples of the sort of metal centers that can be used and examples of linking ligands are given in Figure \(\sf{\PageIndex{2}}\).
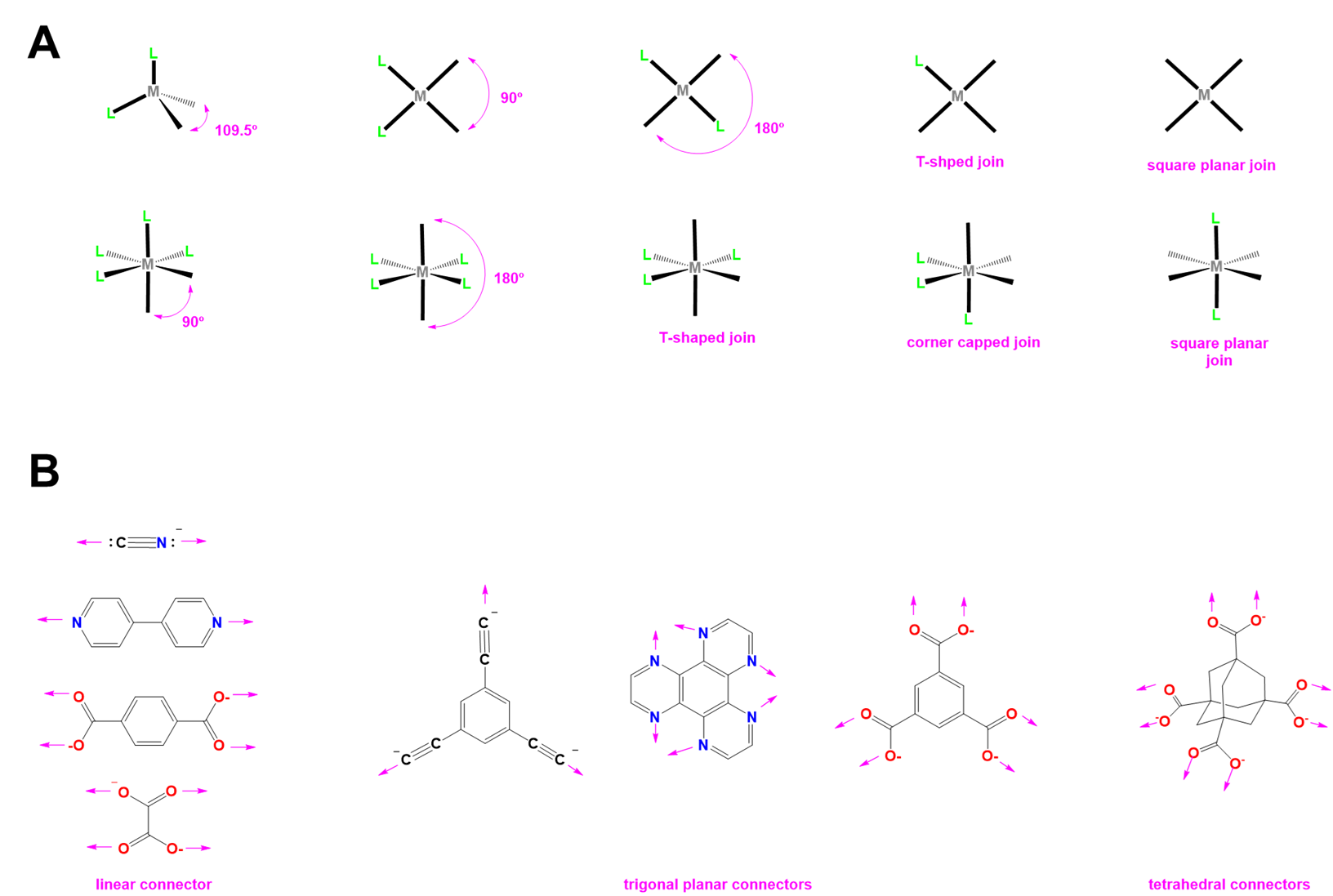
Examples of how the building blocks shown in Figure \(\sf{\PageIndex{2}}\) might be used to prepare molecular polyhedra and coordination networks are depicted schematically as shown in Figure \(\sf{\PageIndex{3}}\). Among the examples shown in Figure \(\sf{\PageIndex{3}}\), those depicted on the right schematize the Hofmann clathrate (top) and Prussian Blue (bottom) coordination networks of Figure \(\sf{\PageIndex{1}}\).
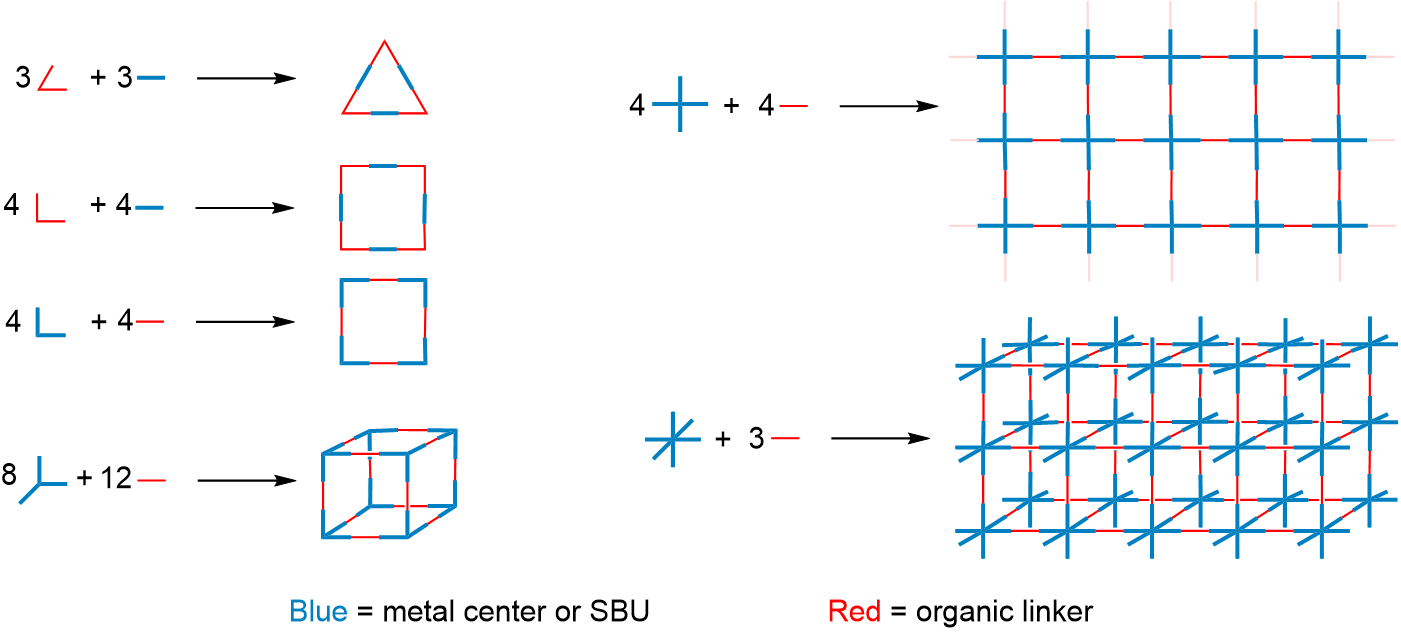

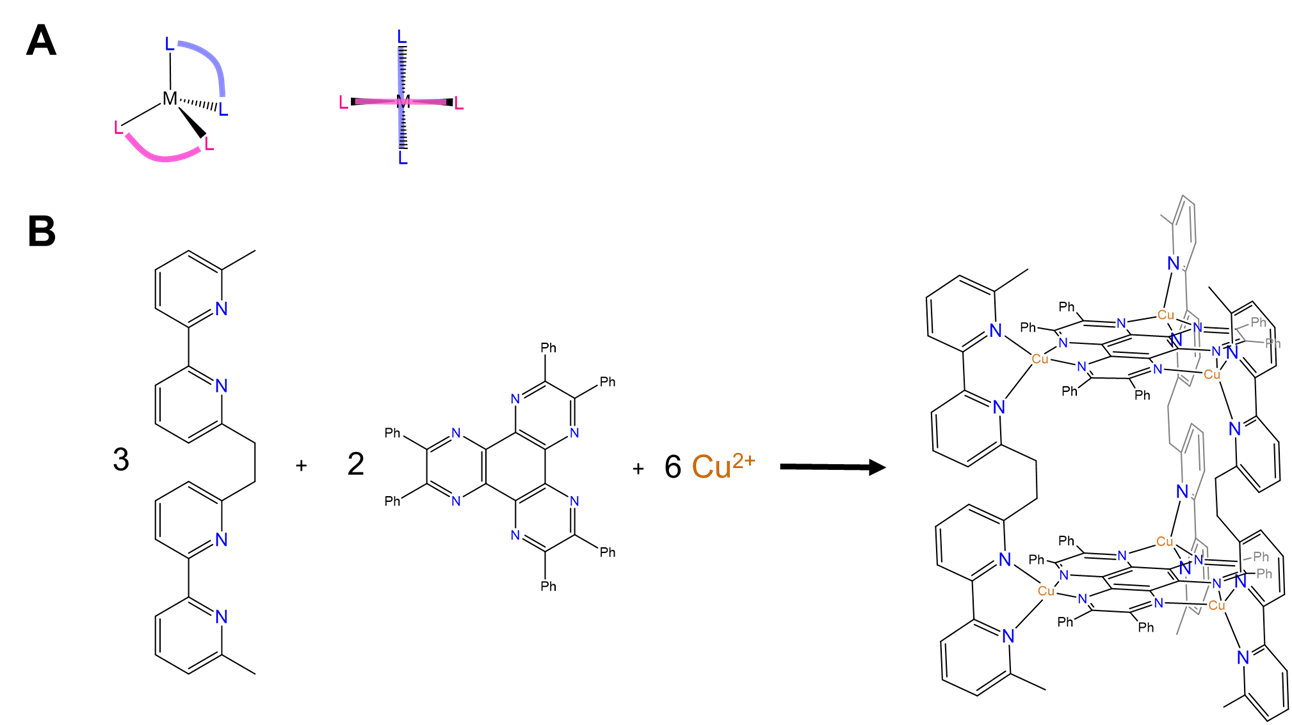
A wider variety of structures are possible than those hinted at by the building blocks and structures shown in Figures \(\sf{\PageIndex{2}}\) and \(\sf{\PageIndex{3}}\). More may be obtained using the principles of molecular structure outlined in the sections on coordination geometry, ligands, and isomerism. In fact, one of the first coordination polyhedra prepared involved Jean-Marie Lehn's recognition that planar bidentate ligands in a tetrahedral bis-chelate are oriented perpendicular to one another, as shown in Figures \(\sf{\PageIndex{5A}}\). This enabled him to prepare a molecular trigonal prism by combining a source of Cu2+ with the trigonal planar and linear-capable organic linkers that bind the Cu2+ in bidentate fashion, as shown in Figure \(\sf{\PageIndex{5B}}\).
Metal Organic Frameworks (MOFs) are porous coordination polymers in which metal "building units" are connected by rigid organic ligand "struts"
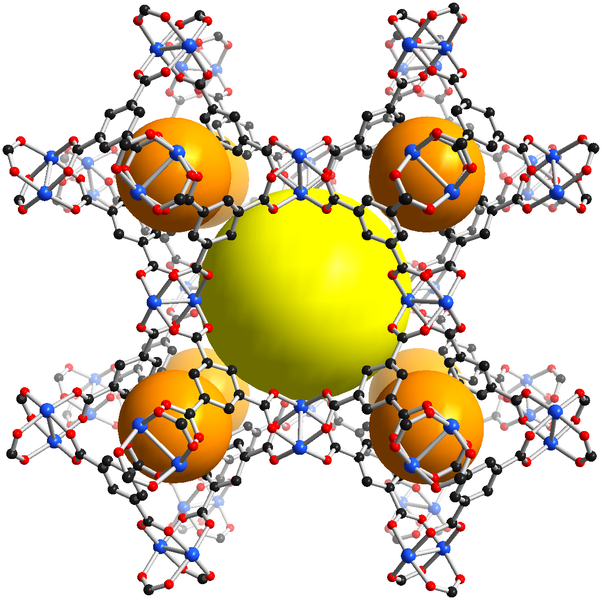
One class of coordination frameworks that has received much attention over the past 25 years is the metal organic frameworks (MOFs). These consist of metal centers linked by rigid organic ligands to give a porous structure similar to those of the aluminosilicate-based zeolites. The structure of one MOF, called MIL-53, is given in Figure \(\sf{\PageIndex{6A}}\).

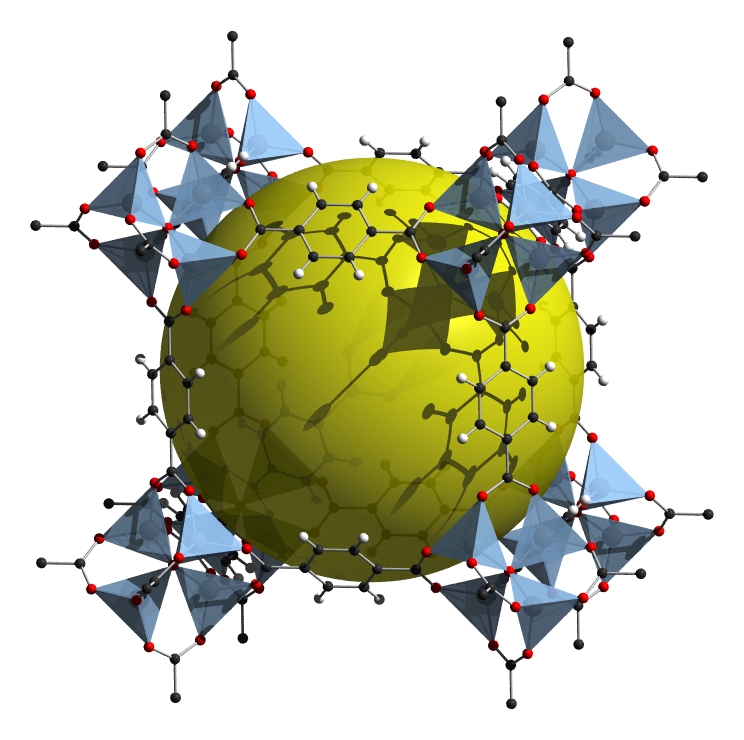
As can be seen from the overall structure of MIL-53 depicted in Figure \(\sf{\PageIndex{6A}}\), the structure possesses large rhombic prismatic voids, represented by the yellow spheres. These voids can in principle be occupied by small molecule substrates. Because of MOF's potential to bind and store substrates, there is considerable interest in developing MOFs that are useful for storing and separating particular gases.
A closer look at the structure of MIL-53 reveals that the carboxylate oxygens in the benzenedicarboxylato (BDU) linkers span two different metal centers rather than binding to only one. As explained in Figure \(\sf{\PageIndex{7}}\), this binding mode enables the formation of rigid stable networks, although it requires the use of metal building blocks in which two or more metal centers are held in close proximity.
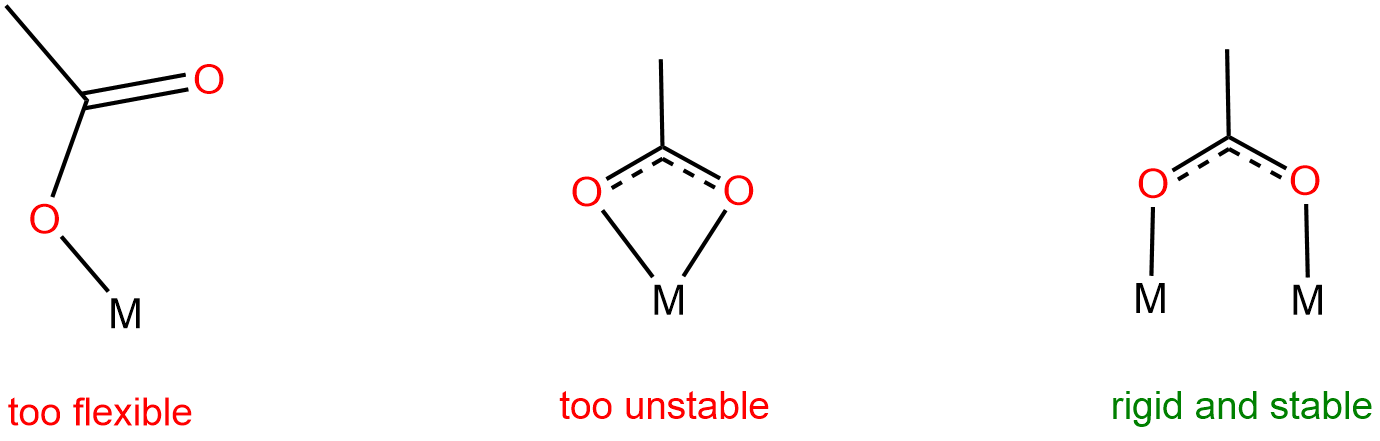
In MIL-53, the metal centers that the DBU ligands coordinate are chains of octahedral metal centers bridged by \(\mu_2\) hydroxo ligands. These chains form spontaneously as the network forms under the high temperature conditions of its synthesis. In other cases, metal secondary building units (SBUs) containing the necessary bridging dicarboxylate ligands are used. One common class of metal SBUs used is the paddlewheel carboxylates shown in Figure \(\sf{\PageIndex{8A}}\). These consist of two metals spanned by a square plane of four carboxylates in a paddlewheel arrangment.
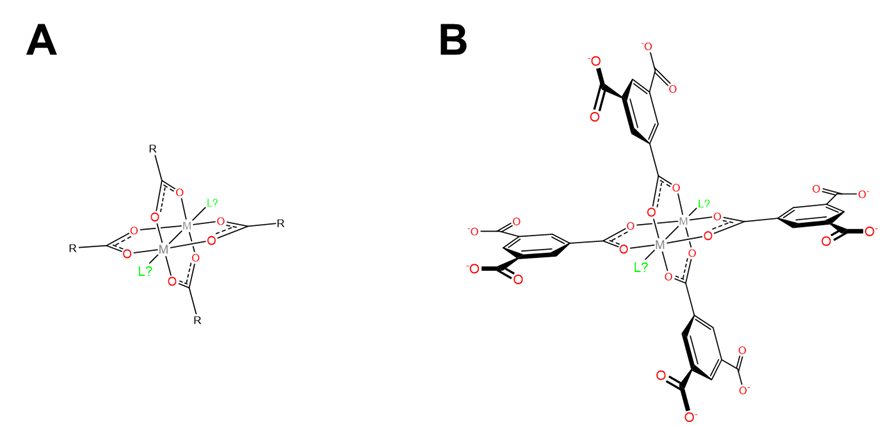
The use of paddlewheel carboxylate metal SBUs and bridging organic carboxylate ligands enables the construction of coordination frameworks like that of HKUST-1 shown in Figure \(\sf{\PageIndex{9}}\). The structure of these networks depends on the geometry of the linking ligand. For instance, in HKUST-1 the 1,3,5-benzenetricarboxylato bridging ligands each link three paddlewheel SBUs to give the cubic network shown in Figure \(\sf{\PageIndex{9}}\). Other organic carboxylate ligands give different network topologies. In addition, the ability of some paddlewheel carboxylates to coordinate ligands perpendicular to the carboxylates, represented by the ligands marked L? in Figure \(\sf{\PageIndex{8}}\), affords additional opportunities to use these networks to bind substrates that can coordinate those sites.
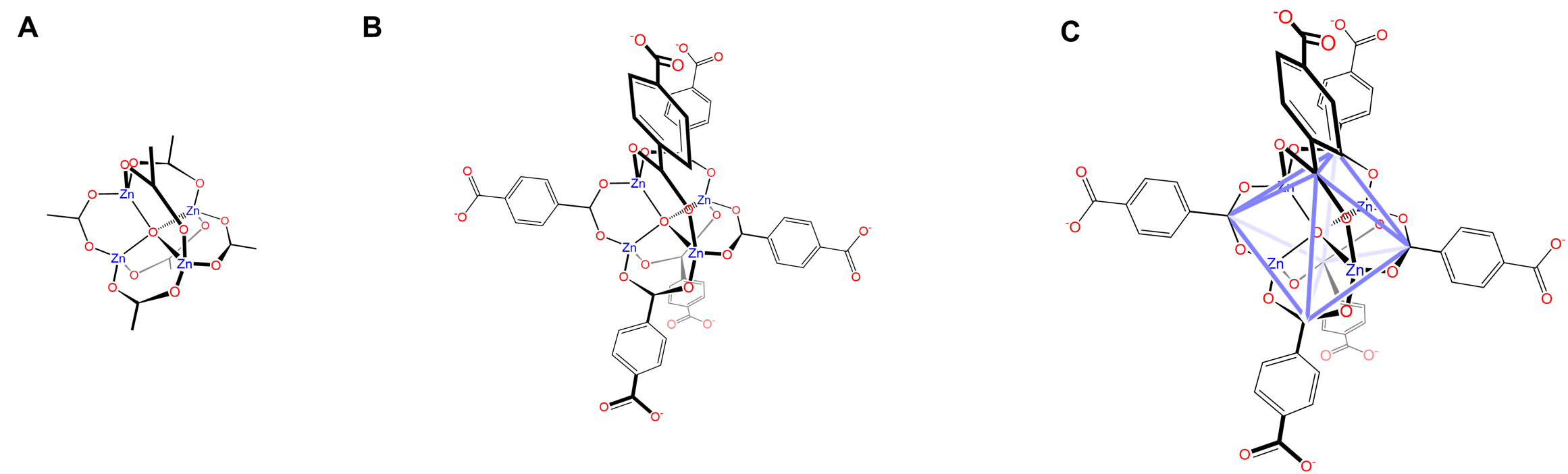
Another common metal SBU is the basic zinc acetate structure shown in Figure \(\sf{\PageIndex{10}}\). As shown in Figure \(\sf{\PageIndex{10A}}\), the structure is analogous to those of the basic beryllium acetates and involves an OZn4 tetrahedron in which the tetrahedrally arranged Zn2+ are connected by carboxylate ligands spanning the tetrahedron's six edges. When these carboxylate ligands are replaced by rigid dicarboxylates (Figure \(\sf{\PageIndex{10B}}\)), the result is that the Zn4O core is surrounded by an octahedral coordination sphere of carboxylate ligands (Figure \(\sf{\PageIndex{10C}}\)).
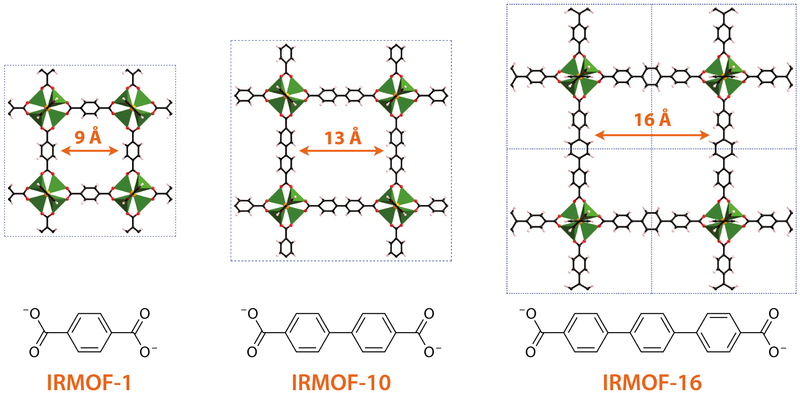
The structure of MOF-5 also illustrates how the size of the pores in an MOF structure may be tailored by lengthening or shortening the organic linker. In particular, from Figure \(\sf{\PageIndex{11}}\) it can be seen that the size of the MOF-5's cubic unit cell depends on the length of the organic portion of the carboxylate linker. In MOF-5 this linker consists of a single benzene unit and gives 9-Angstrom pores, while the use of two and three benzene rings increases the pore size to 13 and 16 Angstroms, respectively, as shown in \(\sf{\PageIndex{12}}\).
References
1. The structure of Prussian blue is rendered from that reported in Buser, H. J.; Schwarzenbach, D.; Petter, W.; Ludi, A., The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3.xH2O. Inorganic Chemistry 1977, 16(11), 2704-2710; to simplify depiction of the coordination framework, water molecules were removed. The structure of the Hofmann-type clathrate is rendered from that reported in Rayner, J. H.; Powell, H. M., 688. Crystal structure of a hydrated nickel cyanide ammoniate. Journal of the Chemical Society (Resumed) 1958, 3412-3418.
2. (a) Fujita, M.; Ogura, K., Transition-metal-directed assembly of well-defined organic architectures possessing large voids: From macrocycles to [2] catenanes. Coordination Chemistry Reviews 1996, 148, 249-264. (b) Leininger, S.; Olenyuk, B.; Stang, P. J., Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chemical Reviews 2000, 100(3), 853-908.
3. Machado, V. G.; Baxter, P. N. W.; Lehn, J.-M., Self-assembly in self-organized inorganic systems: a view of programmed metallosupramolecular architectures. Journal of the Brazilian Chemical Society 2001, 12, 431-462.
4. Yaghi, O. M.; O'Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J., Reticular synthesis and the design of new materials. Nature 2003, 423 (6941), 705-714.

