13.7: The Colligative Properties of Strong Electrolyte Solutions
- Page ID
- 199897
- To understand the factors that determine the solubility of ionic compounds.
The solubility product of an ionic compound describes the concentrations of ions in equilibrium with a solid, but what happens if some of the cations become associated with anions rather than being completely surrounded by solvent? Then predictions of the total solubility of the compound based on the assumption that the solute exists solely as discrete ions would differ substantially from the actual solubility, as would predictions of ionic concentrations. In general, four situations explain why the solubility of a compound may be other than expected: ion pair formation, the incomplete dissociation of molecular solutes, the formation of complex ions, and changes in pH.
Ion-Pair Formation
An ion pair consists of a cation and an anion that are in intimate contact in solution, rather than separated by solvent (Figure \(\PageIndex{1}\)). The ions in an ion pair are held together by the same attractive electrostatic force in ionic solids. As a result, the ions in an ion pair migrate as a single unit, whose net charge is the sum of the charges on the ions. In many ways, we can view an ion pair as a species intermediate between the ionic solid (in which each ion participates in many cation–anion interactions that hold the ions in a rigid array) and the completely dissociated ions in solution (where each is fully surrounded by water molecules and free to migrate independently).
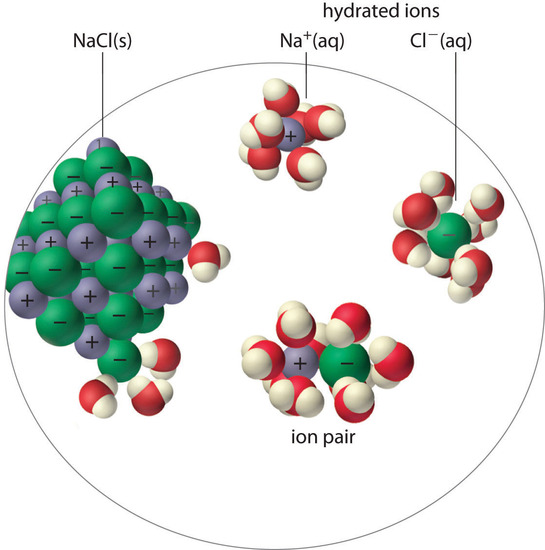
As illustrated for calcium sulfate in the following equation, a second equilibrium must be included to describe the solubility of salts that form ion pairs:
\[\mathrm{CaSO_4(s)}\rightleftharpoons \underbrace{\mathrm{Ca^{2+}}\cdot \mathrm{SO_4^{2-}(aq)}}_ {\textrm{ion pair}} \rightleftharpoons \mathrm{Ca^{2+}(aq)}+\mathrm{SO_4^{2-}(aq)} \label{17.5.3.1} \]
The ion pair is represented by the symbols of the individual ions separated by a dot, which indicates that they are associated in solution. The formation of an ion pair is a dynamic process, just like any other equilibrium, so a particular ion pair may exist only briefly before dissociating into the free ions, each of which may later associate briefly with other ions.
Ion-pair formation can have a major effect on the measured solubility of a salt. For example, the measured Ksp for calcium sulfate is 4.93 × 10−5 at 25°C. The solubility of CaSO4 should be 7.02 × 10−3 M if the only equilibrium involved were as follows:
\[\ce{ CaSO4(s) <=> Ca^{2+}(aq) + SO^{2−}4(aq)} \label{17.5.2} \]
In fact, the experimentally measured solubility of calcium sulfate at 25°C is 1.6 × 10−2 M, almost twice the value predicted from its Ksp. The reason for the discrepancy is that the concentration of ion pairs in a saturated \(\ce{CaSO4}\) solution is almost as high as the concentration of the hydrated ions. Recall that the magnitude of attractive electrostatic interactions is greatest for small, highly charged ions. Hence ion pair formation is most important for salts that contain M2+ and M3+ ions, such as Ca2+ and La3+, and is relatively unimportant for salts that contain monopositive cations, except for the smallest, Li+. We therefore expect a saturated solution of \(\ce{CaSO4}\) to contain a high concentration of ion pairs and its solubility to be greater than predicted from its Ksp.
The formation of ion pairs increases the solubility of a salt.
Incomplete Dissociation
A molecular solute may also be more soluble than predicted by the measured concentrations of ions in solution due to incomplete dissociation. This is particularly common with weak organic acids. Although strong acids (HA) dissociate completely into their constituent ions (H+ and A−) in water, weak acids such as carboxylic acids do not (Ka = 1.5 × 10−5). However, the molecular (undissociated) form of a weak acid (HA) is often quite soluble in water; for example, acetic acid (CH3CO2H) is completely miscible with water. Many carboxylic acids, however, have only limited solubility in water, such as benzoic acid (C6H5CO2H), with Ka = 6.25 × 10−5. Just as with calcium sulfate, we need to include an additional equilibrium to describe the solubility of benzoic acid:
\[ \ce{ C6H5CO2H(s) \rightleftharpoons C6H5CO2H(aq) \rightleftharpoons C6H5CO^{-}2(aq) + H^{+}(aq)} \nonumber \]
In a case like this, measuring only the concentration of the ions grossly underestimates the total concentration of the organic acid in solution. In the case of benzoic acid, for example, the pH of a saturated solution at 25°C is 2.85, corresponding to [H+] = [C6H5CO2−] = 1.4 × 10−3 M. The total concentration of benzoic acid in the solution, however, is 2.8 × 10−2 M. Thus approximately 95% of the benzoic acid in solution is in the form of hydrated neutral molecules—\(C_6H_5CO_2H_{(aq)}\)—and only about 5% is present as the dissociated ions (Figure \(\PageIndex{2}\)).
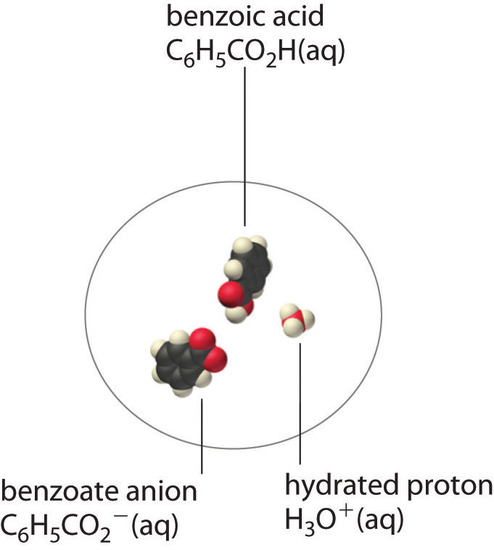
Although ion pairs, such as Ca2+·SO42−, and undissociated electrolytes, such as C6H5CO2H, are both electrically neutral, there is a major difference in the forces responsible for their formation. Simple electrostatic attractive forces between the cation and the anion hold the ion pair together, whereas a polar covalent O−H bond holds together the undissociated electrolyte.
Incomplete dissociation of a molecular solute that is miscible with water can increase the solubility of the solute.
Complex Ion Formation
Previously, you learned that metal ions in aqueous solution are hydrated—that is, surrounded by a shell of usually four or six water molecules. A hydrated ion is one kind of a complex ion (or, simply, complex), a species formed between a central metal ion and one or more surrounding ligands, molecules or ions that contain at least one lone pair of electrons, such as the [Al(H2O)6]3+ ion.
A complex ion forms from a metal ion and a ligand because of a Lewis acid–base interaction. The positively charged metal ion acts as a Lewis acid, and the ligand, with one or more lone pairs of electrons, acts as a Lewis base. Small, highly charged metal ions, such as Cu2+ or Ru3+, have the greatest tendency to act as Lewis acids, and consequently, they have the greatest tendency to form complex ions.
As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated Cu2+ ion {[Cu(H2O)6]2+}. Because it is a stronger base than H2O, ammonia replaces the water molecules in the hydrated ion to form the [Cu(NH3)4(H2O)2]2+ ion. Formation of the [Cu(NH3)4(H2O)2]2+ complex is accompanied by a dramatic color change, as shown in Figure \(\PageIndex{1}\). The solution changes from the light blue of [Cu(H2O)6]2+ to the blue-violet characteristic of the [Cu(NH3)4(H2O)2]2+ ion.
The Formation Constant
The replacement of water molecules from \(\ce{[Cu(H2O)6]^{2+}}\) by ammonia occurs in sequential steps. Omitting the water molecules bound to \(\ce{Cu^{2+}}\) for simplicity, we can write the equilibrium reactions as follows:
\[\begin{align*}\mathrm{Cu^{2+}(aq)}+\mathrm{NH_{3(aq)}}&\rightleftharpoons\mathrm{[Cu(NH_3)]^{2+}_{(aq)}}\quad \quad K_1
\\[4pt] \mathrm{[Cu(NH_3)]^{2+}_{(aq)}}+\mathrm{NH_{3(aq)}}&\rightleftharpoons\mathrm{[Cu(NH_3)_2]^{2+}_{(aq)}} \quad \quad K_2
\\[4pt] \mathrm{[Cu(NH_3)_2]^{2+}_{(aq)}}+\mathrm{NH_{3(aq)}}&\rightleftharpoons\mathrm{[Cu(NH_3)_3]^{2+}_{(aq)}} \quad \quad K_3
\\[4pt] \mathrm{[Cu(NH_3)_3]^{2+}_{(aq)}}+\mathrm{NH_{3(aq)}}&\rightleftharpoons \mathrm{[Cu(NH_3)_4]^{2+}_{(aq)}} \quad \quad K_4 \end{align*} \nonumber \]
The sum of the stepwise reactions is the overall equation for the formation of the complex ion: The hydrated \(\ce{Cu^{2+}}\) ion contains six H2O ligands, but the complex ion that is produced contains only four \(NH_3\) ligands, not six.
\[\ce{Cu^{2+}(aq) + 4NH3(aq) <=> [Cu(NH3)4]^{2+}(aq)} \label{17.3.2} \]
The equilibrium constant for the formation of the complex ion from the hydrated ion is called the formation constant (\(K_f\)). The equilibrium constant expression for \(K_f\) has the same general form as any other equilibrium constant expression. In this case, the expression is as follows:
\[K_\textrm f=\dfrac{\left[[\mathrm{Cu(NH_3)_4}]^{2+}\right]}{[\mathrm{Cu^{2+}}][\mathrm{NH_3}]^4}=2.1\times10^{13}=K_1K_2K_3K_4\label{17.3.3} \]
The formation constant (Kf) has the same general form as any other equilibrium constant expression.
Water, a pure liquid, does not appear explicitly in the equilibrium constant expression, and the hydrated Cu2+(aq) ion is represented as Cu2+ for simplicity. As for any equilibrium, the larger the value of the equilibrium constant (in this case, Kf), the more stable the product. With Kf = 2.1 × 1013, the [Cu(NH3)4(H2O)2]2+ complex ion is very stable. The formation constants for some common complex ions are listed in Table \(\PageIndex{1}\).
| Complex Ion | Equilibrium Equation | Kf | |
|---|---|---|---|
| *Reported values are overall formation constants. Source: Data from Lange’s Handbook of Chemistry, 15th ed. (1999). | |||
| Ammonia Complexes | [Ag(NH3)2]+ | Ag+ + 2NH3 ⇌ [Ag(NH3)2]+ | 1.1 × 107 |
| [Cu(NH3)4]2+ | Cu2+ + 4NH3 ⇌ [Cu(NH3)4]2+ | 2.1 × 1013 | |
| [Ni(NH3)6]2+ | Ni2+ + 6NH3 ⇌ [Ni(NH3)6]2+ | 5.5 × 108 | |
| Cyanide Complexes | [Ag(CN)2]− | Ag+ + 2CN− ⇌ [Ag(CN)2]− | 1.1 × 1018 |
| [Ni(CN)4]2− | Ni2+ + 4CN− ⇌ [Ni(CN)4]2− | 2.2 × 1031 | |
| [Fe(CN)6]3− | Fe3+ + 6CN− ⇌ [Fe(CN)6]3− | 1 × 1042 | |
| Hydroxide Complexes | [Zn(OH)4]2− | Zn2+ + 4OH− ⇌ [Zn(OH)4]2− | 4.6 × 1017 |
| [Cr(OH)4]− | Cr3+ + 4OH− ⇌ [Cr(OH)4]− | 8.0 × 1029 | |
| Halide Complexes | [HgCl4]2− | Hg2+ + 4Cl− ⇌ [HgCl4]2− | 1.2 × 1015 |
| [CdI4]2− | Cd2+ + 4I ⇌ [CdI4]2− | 2.6 × 105 | |
| [AlF6]3− | Al3+ + 6F− ⇌ [AlF6]3− | 6.9 × 1019 | |
| Other Complexes | [Ag(S2O3)2]3− | Ag+ + 2S2O32− ⇌ [Ag(S2O3)2]3− | 2.9 × 1013 |
| [Fe(C2O4)3]3− | Fe3+ + 3C2O42− ⇌ [Fe(C2O4)3]3− | 2.0 × 1020 | |
Example \(\PageIndex{1}\)
If 12.5 g of Cu(NO3)2•6H2O is added to 500 mL of 1.00 M aqueous ammonia, what is the equilibrium concentration of Cu2+(aq)?
Given: mass of Cu2+ salt and volume and concentration of ammonia solution
Asked for: equilibrium concentration of Cu2+(aq)
Strategy:
- Calculate the initial concentration of Cu2+ due to the addition of copper(II) nitrate hexahydrate. Use the stoichiometry of the reaction shown in Equation \(\ref{17.3.2}\) to construct a table showing the initial concentrations, the changes in concentrations, and the final concentrations of all species in solution.
- Substitute the final concentrations into the expression for the formation constant (Equation \(\ref{17.3.3}\)) to calculate the equilibrium concentration of Cu2+(aq).
Solution
Adding an ionic compound that contains Cu2+ to an aqueous ammonia solution will result in the formation of [Cu(NH3)4]2+(aq), as shown in Equation \(\ref{17.3.2}\). We assume that the volume change caused by adding solid copper(II) nitrate to aqueous ammonia is negligible.
A The initial concentration of Cu2+ from the amount of added copper nitrate prior to any reaction is as follows:
\[12.5\mathrm{\;\cancel{g}\;Cu(NO_3)_2}\cdot\mathrm{6H_2O}\left(\dfrac{\textrm{1 mol}}{\textrm{295.65} \cancel{g}} \right )\left(\dfrac{1}{\textrm{500}\; \cancel{mL}} \right )\left(\dfrac{\textrm{1000}\; \cancel{mL}}{\textrm{1 L}} \right )=\textrm{0.0846 M} \nonumber \]
Because the stoichiometry of the reaction is four NH3 to one Cu2+, the amount of NH3 required to react completely with the Cu2+ is 4(0.0846) = 0.338 M. The concentration of ammonia after complete reaction is 1.00 M − 0.338 M = 0.66 M. These results are summarized in the first two lines of the following table. Because the equilibrium constant for the reaction is large (2.1 × 1013), the equilibrium will lie far to the right. Thus we will assume that the formation of [Cu(NH3)4]2+ in the first step is complete and allow some of it to dissociate into Cu2+ and NH3 until equilibrium has been reached. If we define x as the amount of Cu2+ produced by the dissociation reaction, then the stoichiometry of the reaction tells us that the change in the concentration of [Cu(NH3)4]2+ is −x, and the change in the concentration of ammonia is +4x, as indicated in the table. The final concentrations of all species (in the bottom row of the table) are the sums of the concentrations after complete reaction and the changes in concentrations.
\[\ce{Cu^{2+} + 4NH3 <=> [Cu(NH3)4]^{2+}} \nonumber \]
| [Cu2+] | [NH3] | [[Cu(NH3)4]2+] | |
|---|---|---|---|
| initial | 0.0846 | 1.00 | 0 |
| after complete reaction | 0 | 0.66 | 0.0846 |
| change | +x | +4x | −x |
| final | x | 0.66 + 4x | 0.0846 − x |
B Substituting the final concentrations into the expression for the formation constant (Equation \(\ref{17.3.3}\)) and assuming that \(x \ll 0.0846\), which allows us to remove x from the sum and difference,
\[\begin{align*}K_\textrm f&=\dfrac{\left[[\mathrm{Cu(NH_3)_4}]^{2+}\right]}{[\mathrm{Cu^{2+}}][\mathrm{NH_3}]^4}=\dfrac{0.0846-x}{x(0.66+4x)^4}\approx\dfrac{0.0846}{x(0.66)^4}=2.1\times10^{13}
\\x&=2.1\times 10^{-14}\end{align*} \nonumber \]
The value of \(x\) indicates that our assumption was justified. The equilibrium concentration of \(\ce{Cu^{2+}(aq)}\) in a 1.00 M ammonia solution is therefore \(2.1 \times 10^{−14} M\).
The ferrocyanide ion \(\ce{[Fe(CN)6]^{4−}}\) is very stable, with a Kf = 1 × 1035. Calculate the concentration of cyanide ion in equilibrium with a 0.65 M solution of \(\ce{K4[Fe(CN)6]}\).
- Answer
-
2 × 10−6 M
The Effect of the Formation of Complex Ions on Solubility
What happens to the solubility of a sparingly soluble salt if a ligand that forms a stable complex ion is added to the solution? One such example occurs in conventional black-and-white photography. Recall that black-and-white photographic film contains light-sensitive microcrystals of AgBr, or mixtures of AgBr and other silver halides. AgBr is a sparingly soluble salt, with a Ksp of 5.35 × 10−13 at 25°C. When the shutter of the camera opens, the light from the object being photographed strikes some of the crystals on the film and initiates a photochemical reaction that converts AgBr to black Ag metal. Well-formed, stable negative images appear in tones of gray, corresponding to the number of grains of AgBr converted, with the areas exposed to the most light being darkest. To fix the image and prevent more AgBr crystals from being converted to Ag metal during processing of the film, the unreacted \(\ce{AgBr}\) on the film is removed using a complexation reaction to dissolve the sparingly soluble salt.
The reaction for the dissolution of silver bromide is as follows:
\[\ce{AgBr(s) <=> Ag^{+}(aq) + Br^{-}(aq)} \nonumber \]
with \(K_{sp} = 5.35 \times 10^{−13}\) at 25°C.
The equilibrium lies far to the left, and the equilibrium concentrations of Ag+ and Br− ions are very low (7.31 × 10−7 M). As a result, removing unreacted AgBr from even a single roll of film using pure water would require tens of thousands of liters of water and a great deal of time. Le Chatelier’s principle tells us, however, that we can drive the reaction to the right by removing one of the products, which will cause more AgBr to dissolve. Bromide ion is difficult to remove chemically, but silver ion forms a variety of stable two-coordinate complexes with neutral ligands, such as ammonia, or with anionic ligands, such as cyanide or thiosulfate (S2O32−). In photographic processing, excess AgBr is dissolved using a concentrated solution of sodium thiosulfate.
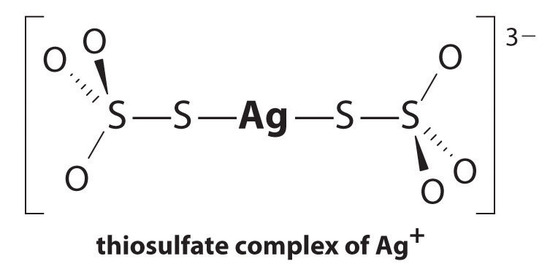
The reaction of Ag+ with thiosulfate is as follows:
\[\ce{Ag^{+}(aq) + 2S2O^{2-}3(aq) <=> [Ag(S2O3)2]^{3-}(aq)} \nonumber \]
with \(K_f = 2.9 \times 10^{13}\).
The magnitude of the equilibrium constant indicates that almost all \(\ce{Ag^{+}}\) ions in solution will be immediately complexed by thiosulfate to form [Ag(S2O3)2]3−. We can see the effect of thiosulfate on the solubility of AgBr by writing the appropriate reactions and adding them together:
\\ \mathrm{Ag^+(aq)}+\mathrm{2S_2O_3^{2-}(aq)}\rightleftharpoons\mathrm{[Ag(S_2O_3)_2]^{3-}(aq)}\hspace{3mm}K_\textrm f&=2.9\times10^{13}
\\ \mathrm{AgBr(s)}+\mathrm{2S_2O_3^{2-}(aq)}\rightleftharpoons\mathrm{[Ag(S_2O_3)_2]^{3-}(aq)}+\mathrm{Br^-(aq)}\hspace{3mm}K&=K_{\textrm{sp}}K_{\textrm f}=15\end{align} \label{17.3.6}\)
Comparing K with Ksp shows that the formation of the complex ion increases the solubility of AgBr by approximately 3 × 1013. The dramatic increase in solubility combined with the low cost and the low toxicity explains why sodium thiosulfate is almost universally used for developing black-and-white film. If desired, the silver can be recovered from the thiosulfate solution using any of several methods and recycled.
If a complex ion has a large Kf, the formation of a complex ion can dramatically increase the solubility of sparingly soluble salts.
Due to the common ion effect, we might expect a salt such as AgCl to be much less soluble in a concentrated solution of KCl than in water. Such an assumption would be incorrect, however, because it ignores the fact that silver ion tends to form a two-coordinate complex with chloride ions (AgCl2−). Calculate the solubility of AgCl in each situation:
- in pure water
- in 1.0 M KCl solution, ignoring the formation of any complex ions
- the same solution as in part (b) except taking the formation of complex ions into account, assuming that AgCl2− is the only Ag+ complex that forms in significant concentrations
At 25°C, Ksp = 1.77 × 10−10 for AgCl and Kf = 1.1 × 105 for AgCl2−.
Given: Ksp of AgCl, Kf of AgCl2−, and KCl concentration
Asked for: solubility of AgCl in water and in KCl solution with and without the formation of complex ions
Strategy:
- Write the solubility product expression for AgCl and calculate the concentration of Ag+ and Cl− in water.
- Calculate the concentration of Ag+ in the KCl solution.
- Write balanced chemical equations for the dissolution of AgCl and for the formation of the AgCl2− complex. Add the two equations and calculate the equilibrium constant for the overall equilibrium.
- Write the equilibrium constant expression for the overall reaction. Solve for the concentration of the complex ion.
Solution
- A If we let x equal the solubility of AgCl, then at equilibrium [Ag+] = [Cl−] = x M. Substituting this value into the solubility product expression,
Thus the solubility of AgCl in pure water at 25°C is 1.33 × 10−5 M.
- B If x equals the solubility of AgCl in the KCl solution, then at equilibrium [Ag+] = x M and [Cl−] = (1.0 + x) M. Substituting these values into the solubility product expression and assuming that x << 1.0,
If the common ion effect were the only important factor, we would predict that AgCl is approximately five orders of magnitude less soluble in a 1.0 M KCl solution than in water.
- C To account for the effects of the formation of complex ions, we must first write the equilibrium equations for both the dissolution and the formation of complex ions. Adding the equations corresponding to Ksp and Kf gives us an equation that describes the dissolution of AgCl in a KCl solution. The equilibrium constant for the reaction is therefore the product of Ksp and Kf:
\\ \mathrm{Ag^+(aq)}+\mathrm{2Cl^{-}}\rightleftharpoons\mathrm{[AgCl_2]^{-}}\hspace{3mm}K_\textrm f&=1.1\times10^{5}
\\ \mathrm{AgCl(s)}+\mathrm{Cl^{-}}\rightleftharpoons\mathrm{[AgCl_2]^{-}}\hspace{3mm}K&=K_{\textrm{sp}}K_{\textrm f}=1.9\times10^{-5}\end{align}\)
D If we let x equal the solubility of AgCl in the KCl solution, then at equilibrium [AgCl2−] = x and [Cl−] = 1.0 − x. Substituting these quantities into the equilibrium constant expression for the net reaction and assuming that x << 1.0,
That is, AgCl dissolves in 1.0 M KCl to produce a 1.9 × 10−5 M solution of the AgCl2− complex ion. Thus we predict that AgCl has approximately the same solubility in a 1.0 M KCl solution as it does in pure water, which is 105 times greater than that predicted based on the common ion effect. (In fact, the measured solubility of AgCl in 1.0 M KCl is almost a factor of 10 greater than that in pure water, largely due to the formation of other chloride-containing complexes.)
Calculate the solubility of mercury(II) iodide (\(\ce{HgI2}\)) in each situation:
- pure water
- a 3.0 M solution of NaI, assuming [HgI4]2− is the only Hg-containing species present in significant amounts
Ksp = 2.9 × 10−29 for HgI2 and Kf = 6.8 × 1029 for [HgI4]2−.
Answer
- 1.9 × 10−10 M
- 1.4 M
Solubility of Complex Ions: Solubility of Complex Ions(opens in new window) [youtu.be]
Complexing agents, molecules or ions that increase the solubility of metal salts by forming soluble metal complexes, are common components of laundry detergents. Long-chain carboxylic acids, the major components of soaps, form insoluble salts with Ca2+ and Mg2+, which are present in high concentrations in “hard” water. The precipitation of these salts produces a bathtub ring and gives a gray tinge to clothing. Adding a complexing agent such as pyrophosphate (O3POPO34−, or P2O74−) or triphosphate (P3O105−) to detergents prevents the magnesium and calcium salts from precipitating because the equilibrium constant for complex-ion formation is large:
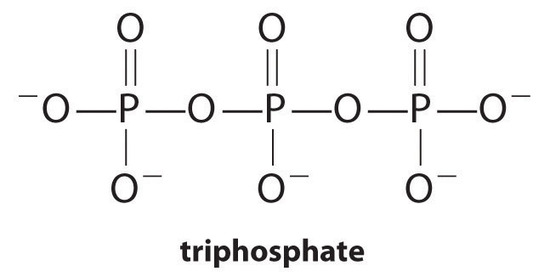
\[\ce{Ca^{2+}(aq) + O_3POPO^{4−}4(aq) <=> [Ca(O_3POPO_3)]^{2−}(aq)} \label{17.3.7a} \]
with \(K_f = 4\times 10^4\).
However, phosphates can cause environmental damage by promoting eutrophication, the growth of excessive amounts of algae in a body of water, which can eventually lead to large decreases in levels of dissolved oxygen that kill fish and other aquatic organisms. Consequently, many states in the United States have banned the use of phosphate-containing detergents, and France has banned their use beginning in 2007. “Phosphate-free” detergents contain different kinds of complexing agents, such as derivatives of acetic acid or other carboxylic acids. The development of phosphate substitutes is an area of intense research.
Commercial water softeners also use a complexing agent to treat hard water by passing the water over ion-exchange resins, which are complex sodium salts. When water flows over the resin, sodium ion is dissolved, and insoluble salts precipitate onto the resin surface. Water treated in this way has a saltier taste due to the presence of Na+, but it contains fewer dissolved minerals.
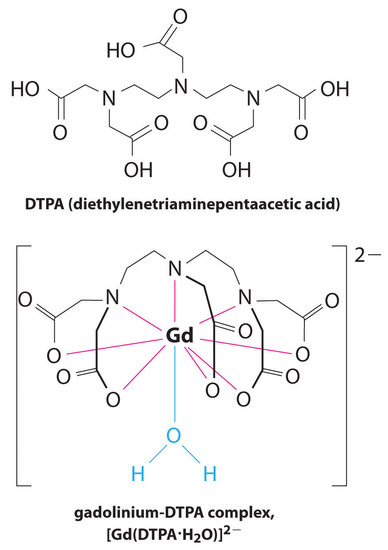
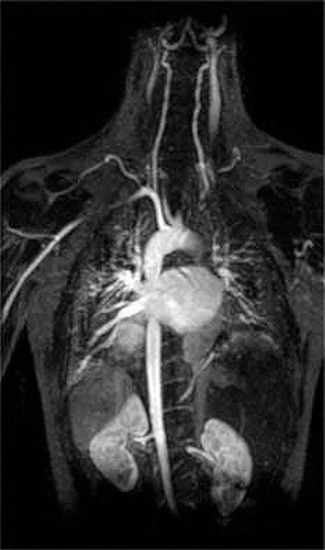
Another application of complexing agents is found in medicine. Unlike x-rays, magnetic resonance imaging (MRI) can give relatively good images of soft tissues such as internal organs. MRI is based on the magnetic properties of the 1H nucleus of hydrogen atoms in water, which is a major component of soft tissues. Because the properties of water do not depend very much on whether it is inside a cell or in the blood, it is hard to get detailed images of these tissues that have good contrast. To solve this problem, scientists have developed a class of metal complexes known as “MRI contrast agents.” Injecting an MRI contrast agent into a patient selectively affects the magnetic properties of water in cells of normal tissues, in tumors, or in blood vessels and allows doctors to “see” each of these separately (Figure \(\PageIndex{4}\)). One of the most important metal ions for this application is Gd3+, which with seven unpaired electrons is highly paramagnetic. Because Gd3+(aq) is quite toxic, it must be administered as a very stable complex that does not dissociate in the body and can be excreted intact by the kidneys. The complexing agents used for gadolinium are ligands such as DTPA5− (diethylene triamine pentaacetic acid), whose fully protonated form is shown here.
Summary
Ion-pair formation, the incomplete dissociation of molecular solutes, the formation of complex ions, and changes in pH all affect solubility. There are four explanations why the solubility of a compound can differ from the solubility indicated by the concentrations of ions: (1) ion pair formation, in which an anion and a cation are in intimate contact in solution and not separated by solvent, (2) the incomplete dissociation of molecular solutes, (3) the formation of complex ions, and (4) changes in pH. An ion pair is held together by electrostatic attractive forces between the cation and the anion, whereas incomplete dissociation results from intramolecular forces, such as polar covalent O–H bonds.
The formation of complex ions can substantially increase the solubility of sparingly soluble salts if the complex ion has a large Kf. A complex ion is a species formed between a central metal ion and one or more surrounding ligands, molecules or ions that contain at least one lone pair of electrons. Small, highly charged metal ions have the greatest tendency to act as Lewis acids and form complex ions. The equilibrium constant for the formation of the complex ion is the formation constant (Kf). The formation of a complex ion by adding a complexing agent increases the solubility of a compound.

