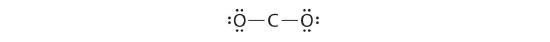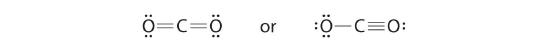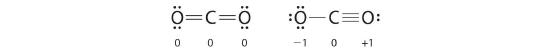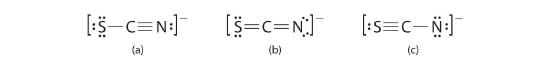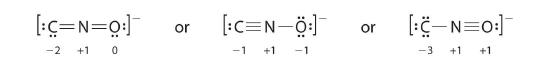2.3 Formal Charges
- Page ID
- 44170
Objectives
After completing this section, you should be able to
- determine which atoms, if any, of a given simple compound (e.g., HNO3, CH2=N=N, CH3-NC) carry formal charges, and apply the principles used to more complex examples. [The Lewis structure, Kekulé structure or molecular formula would normally be provided.]
- draw the Lewis structure, the Kekulé structure, or both, of a compound of known molecular formula in which certain atoms possess a formal charge
Key Terms
Make certain that you can define, and use in context, each of the key terms listed below.
- dipolar
- formal charge
Study Notes
Too much emphasis can easily be placed on the concept of formal charge, and the mathematical approach used in the textbook is hard to justify. In this course, you will certainly need to be able to recognize whether a given species carries a charge (i.e., is an ion), but you will not often encounter dipolar molecules, such as the textbook example nitromethane (see page 42).
Formal Charges
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH2O, but not every Lewis structure may be equally reasonable. In these situations, we can choose the most stable Lewis structure by considering the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure.![]()
To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:![]()
![]()
- Nonbonding electrons are assigned to the atom on which they are located.
- Bonding electrons are divided equally between the bonded atoms.
For each atom, we then compute a formal charge:
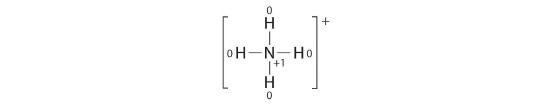
To illustrate this method, let’s calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows:
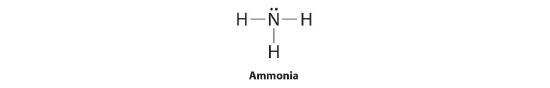
A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e− + (6 bonding e−/2)]. Substituting into Equation 5.3.1, we obtain
A neutral hydrogen atom has one valence electron. Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (2 bonding e−/2)]. Using Equation 4.4.1 to calculate the formal charge on hydrogen, we obtain
The hydrogen atoms in ammonia have the same number of electrons as neutral hydrogen atoms, and so their formal charge is also zero. Adding together the formal charges should give us the overall charge on the molecule or ion. In this example, the nitrogen and each hydrogen has a formal charge of zero. When summed the overall charge is zero, which is consistent with the overall charge on the NH3 molecule.![]()
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. In cases where there are positive or negative formal charges on various atoms, stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. The next example further demonstrates how to calculate formal charges.
Example 2.3.1
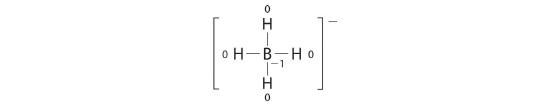
Calculate the formal charges on each atom in the NH4+ ion.
Given: chemical species
Asked for: formal charges
Strategy:
Identify the number of valence electrons in each atom in the NH4+ ion. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation 4.4.1 to calculate the formal charge on each atom.
Solution:
The Lewis electron structure for the NH4+ ion is as follows:

The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen atom is therefore
The formal charges on the atoms in the NH4+ ion are thus
Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1.
Common bonding patterns in organic structures
The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. But as you can imagine, these methods become unreasonably tedious and time-consuming when you start dealing with larger structures. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons.
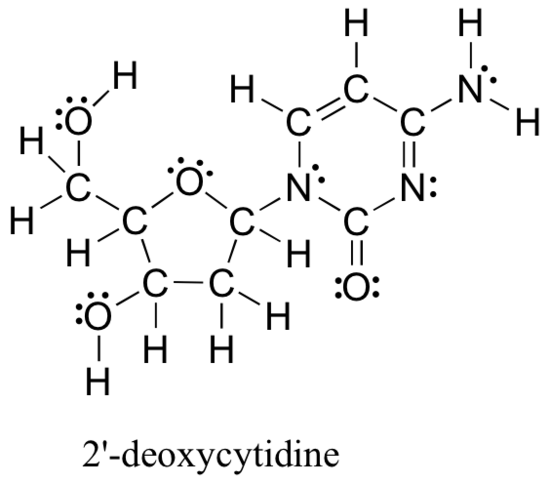
And yet, as organic chemists, and especially as organic chemists dealing with biological molecules, you will be expected soon to draw the structure of large molecules such as this on a regular basis. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns.
Let’s start with carbon, the most important element for organic chemists. Carbon is said to be tetravalent, meaning that it tends to form four bonds. If you look at the simple structures of methane, methanol, ethane, ethene, and ethyne in the figures from the previous section, you should quickly recognize that in each molecule, the carbon atom has four bonds, and a formal charge of zero.
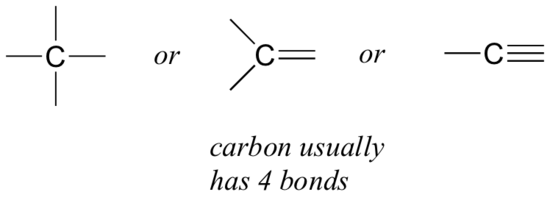
This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions.
In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. If a carbon has only three bonds and an unfilled valence shell (in other words, if it does not fulfill the octet rule), it will have a positive formal charge.
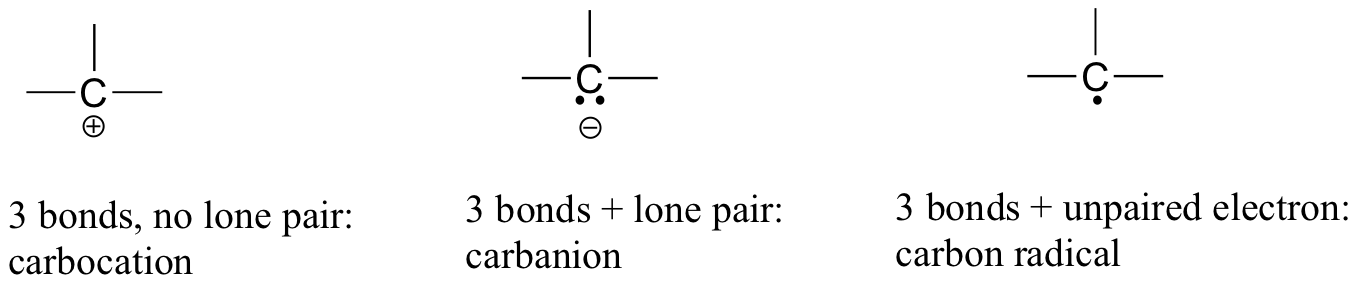
If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. Another possibility is a carbon with three bonds and a single, unpaired (free radical) electron: in this case, the carbon has a formal charge of zero. (One last possibility is a highly reactive species called a ‘carbene’, in which a carbon has two bonds and one lone pair of electrons, giving it a formal charge of zero. You may encounter carbenes in more advanced chemistry courses, but they will not be discussed any further in this book).
You should certainly use the methods you have learned to check that these formal charges are correct for the examples given above. More importantly, you will need, before you progress much further in your study of organic chemistry, to simply recognize these patterns (and the patterns described below for other atoms) and be able to identify carbons that bear positive and negative formal charges by a quick inspection.
The pattern for hydrogens is easy: hydrogen atoms have only one bond, and no formal charge. The exceptions to this rule are the proton, H+, and the hydride ion, H-, which is a proton plus two electrons. Because we are concentrating in this book on organic chemistry as applied to living things, however, we will not be seeing ‘naked’ protons and hydrides as such, because they are too reactive to be present in that form in aqueous solution. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge.
Let us next turn to oxygen atoms. Typically, you will see an oxygen bonding in three ways, all of which fulfill the octet rule.
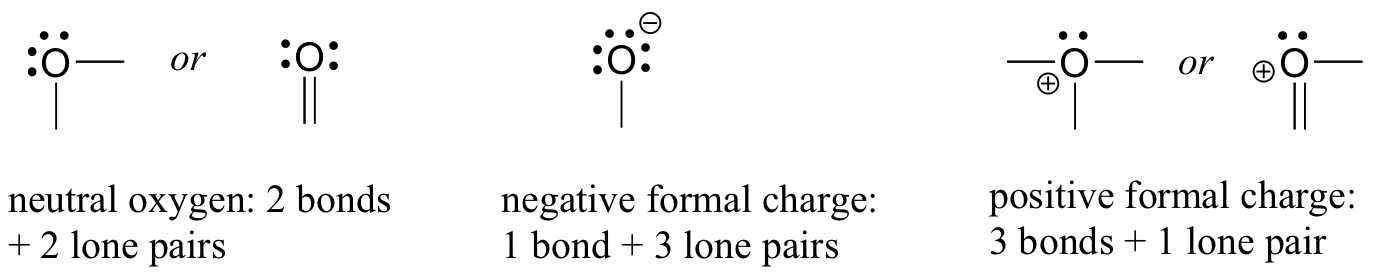
If it has two bonds and two lone pairs, as in water, it will have a formal charge of zero. If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1.
When we get to our discussion of free radical chemistry in chapter 17, we will see other possibilities, such as where an oxygen atom has one bond, one lone pair, and one unpaired (free radical) electron, giving it a formal charge of zero. For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17.
Nitrogen has two major bonding patterns, both of which fulfill the octet rule:
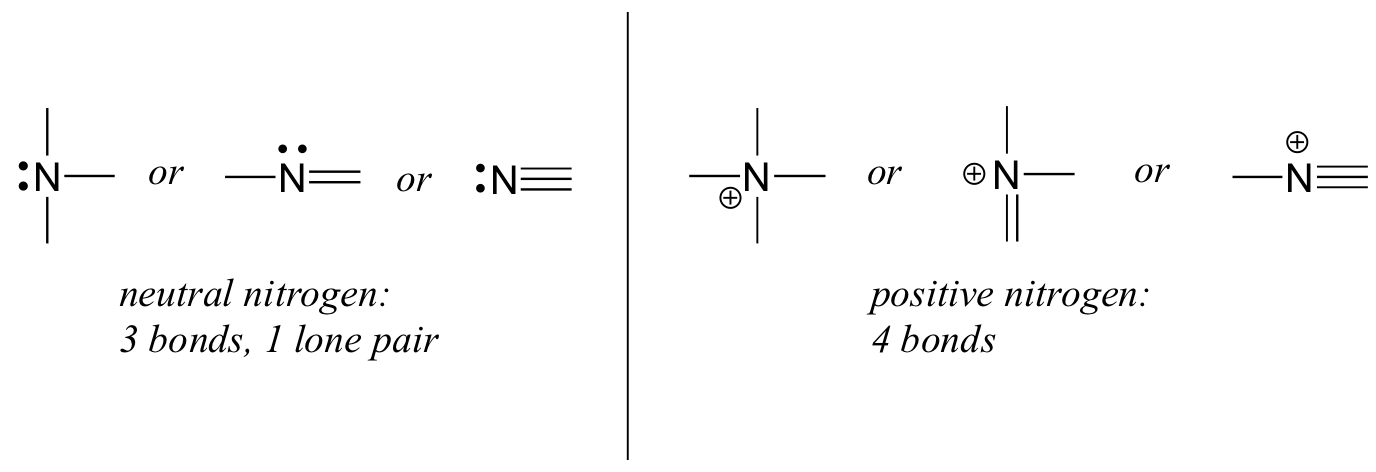
If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If it has four bonds (and no lone pair), it has a formal charge of +1. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs.
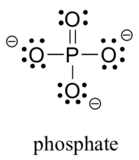
Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero. Remember that elements in the third row of the periodic table have d orbitals in their valence shell as well as s and p orbitals, and thus are not bound by the octet rule.
Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. Halogens in organic compounds usually are seen with one bond, three lone pairs, and a formal charge of zero. Sometimes, especially in the case of bromine, we will encounter reactive species in which the halogen has two bonds (usually in a three-membered ring), two lone pairs, and a formal charge of +1.

These rules, if learned and internalized so that you don’t even need to think about them, will allow you to draw large organic structures, complete with formal charges, quite quickly.
Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). Occasionally, though, lone pairs are drawn if doing so helps to make an explanation more clear.
Using Formal Charges to Distinguish between Lewis Structures
As an example of how formal charges can be used to determine the most stable Lewis structure for a substance, we can compare two possible structures for CO2. Both structures conform to the rules for Lewis electron structures.![]()
CO2
1. C is less electronegative than O, so it is the central atom.![]()
2. C has 4 valence electrons and each O has 6 valence electrons, for a total of 16 valence electrons.![]()
3. Placing one electron pair between the C and each O gives O–C–O, with 12 electrons left over.![]()
4. Dividing the remaining electrons between the O atoms gives three lone pairs on each atom:
This structure has an octet of electrons around each O atom but only 4 electrons around the C atom.
5. No electrons are left for the central atom.![]()
6. To give the carbon atom an octet of electrons, we can convert two of the lone pairs on the oxygen atoms to bonding electron pairs. There are, however, two ways to do this. We can either take one electron pair from each oxygen to form a symmetrical structure or take both electron pairs from a single oxygen atom to give an asymmetrical structure:
Both Lewis electron structures give all three atoms an octet. How do we decide between these two possibilities? The formal charges for the two Lewis electron structures of CO2 are as follows:
Both Lewis structures have a net formal charge of zero, but the structure on the right has a +1 charge on the more electronegative atom (O). Thus the symmetrical Lewis structure on the left is predicted to be more stable, and it is, in fact, the structure observed experimentally. Remember, though, that formal charges do not represent the actual charges on atoms in a molecule or ion. They are used simply as a bookkeeping method for predicting the most stable Lewis structure for a compound.![]()
Example 2.3.2
The thiocyanate ion (SCN−), which is used in printing and as a corrosion inhibitor against acidic gases, has at least two possible Lewis electron structures. Draw two possible structures, assign formal charges on all atoms in both, and decide which is the preferred arrangement of electrons.
Given: chemical species
Asked for: Lewis electron structures, formal charges, and preferred arrangement
Strategy:
A Use the step-by-step procedure to write two plausible Lewis electron structures for SCN−.
B Calculate the formal charge on each atom using Equation 4.4.1.
C Predict which structure is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms present.
Solution:
A Possible Lewis structures for the SCN− ion are as follows:
B We must calculate the formal charges on each atom to identify the more stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero. Continuing with sulfur, we observe that in (a) the sulfur atom shares one bonding pair and has three lone pairs and has a total of six valence electrons. The formal charge on the sulfur atom is therefore
C Which structure is preferred? Structure (b) is preferred because the negative charge is on the more electronegative atom (N), and it has lower formal charges on each atom as compared to structure (c): 0, −1 versus +1, −2.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)