2.1 Polar Covalent Bonds: Electronegativity
- Page ID
- 44168
Objectives
After completing this section, you should be able to
- describe how differences in electronegativity give rise to bond polarity.
- arrange a given series of the elements most often encountered in organic chemistry (C, H, O, S, P and the halogens) in order of increasing or decreasing electronegativity, without referring to a table of electronegativities.
- predict the positive and negative ends of a given bond formed between any two of the elements listed in Objective 2, above, without the use of a table of electronegativities or a periodic table.
- predict the positive and negative ends of a given bond formed between any two elements not listed in Objective 2, above, using a periodic table.
Key Terms
Make certain that you can define, and use in context, each of the key terms listed below.
- electronegativity
- inductive effect
- polar colvalent bond
Study Notes
Students often wonder why it is important to be able to tell whether or not a given bond is polar, and why they need to know which atoms carry a partial positive charge and which a partial negative charge. Consider the chloromethane (CH3Cl) molecule (see page 37 of the textbook). As you see, the carbon atom is shown as carrying a partial positive charge. Now, recall that opposite charges attract. Thus, it seems reasonable that the slightly positive carbon atom in chloromethane should be susceptible to attack by a negatively charged species, such as the hydroxide ion, OH−. This theory is borne out in practice: hydroxide ions react with chloromethane by attacking the slightly positive carbon atom in the latter. It is often possible to rationalize chemical reactions in this manner, and you will find a knowledge of bond polarity indispensible when you start to write reaction mechanisms. Note: Because of the small difference in electronegativity between carbon and hydrogen, the C-H bond is normally assumed to be nonpolar.
Formal Charges
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH2O, but not every Lewis structure may be equally reasonable. In these situations, we can choose the most stable Lewis structure by considering the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure.
To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:
- Nonbonding electrons are assigned to the atom on which they are located.
- Bonding electrons are divided equally between the bonded atoms.
For each atom, we then compute a formal charge:
\( \begin{matrix}
formal\; charge= & valence\; e^{-}- & \left ( non-bonding\; e^{-}+\frac{bonding\;e^{-}}{2} \right )\\
& ^{\left ( free\; atom \right )} & ^{\left ( atom\; in\; Lewis\; structure \right )}
\end{matrix} \tag{8.5.1} \) (atom in Lewis structure)
To illustrate this method, let’s calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows:
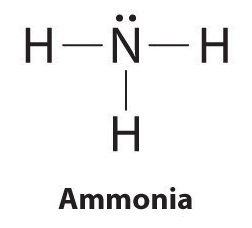
A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e− + (6 bonding e−/2)]. Substituting into Equation 2.1.1, we obtain
\[ formal\; charge\left ( N \right )=5\; valence\; e^{-}-\left ( 2\; non-bonding\; e^{-} +\frac{6\; bonding\; e^{-}}{2} \right )=0 \tag{2.1.1}\]
A neutral hydrogen atom has one valence electron. Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (2 bonding e−/2)]. Using Equation 2.1.1 to calculate the formal charge on hydrogen, we obtain
\[ formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\frac{2\; bonding\; e^{-}}{2} \right )=0 \tag{8.5.3}\]
The hydrogen atoms in ammonia have the same number of electrons as neutral hydrogen atoms, and so their formal charge is also zero. Adding together the formal charges should give us the overall charge on the molecule or ion. In this example, the nitrogen and each hydrogen has a formal charge of zero. When summed the overall charge is zero, which is consistent with the overall charge on the NH3 molecule.
Note
An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species.
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. In cases where there are positive or negative formal charges on various atoms, stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. The next example further demonstrates how to calculate formal charges.
Example 2.1.1
Calculate the formal charges on each atom in the NH4+ ion.
Given: chemical species
Asked for: formal charges
Strategy:
Identify the number of valence electrons in each atom in the NH4+ ion. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation 2.1.1 to calculate the formal charge on each atom.
Solution:
The Lewis electron structure for the NH4+ ion is as follows:
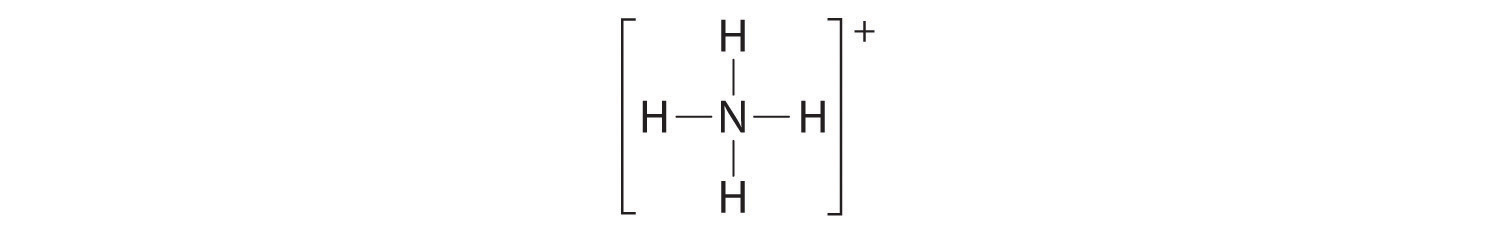
The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 8.5.1, the formal charge on the nitrogen atom is therefore
\[ formal\; charge\left ( N \right )=5-\left ( 0+\frac{8}{2} \right )=0 \]
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen atom is therefore
\[ formal\; charge\left ( H \right )=1-\left ( 0+\frac{2}{2} \right )=0 \]
The formal charges on the atoms in the NH4+ ion are thus
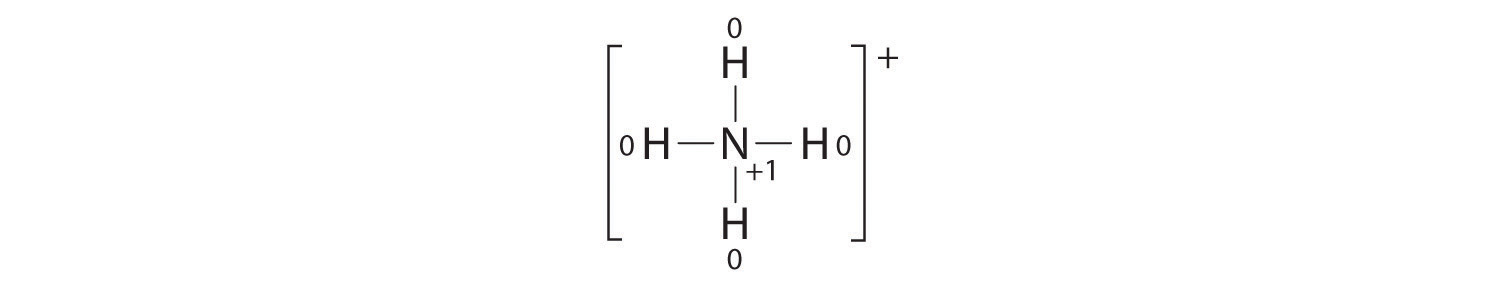
Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1.
Exercise 2.1.1
Write the formal charges on all atoms in BH4−.
Answer:
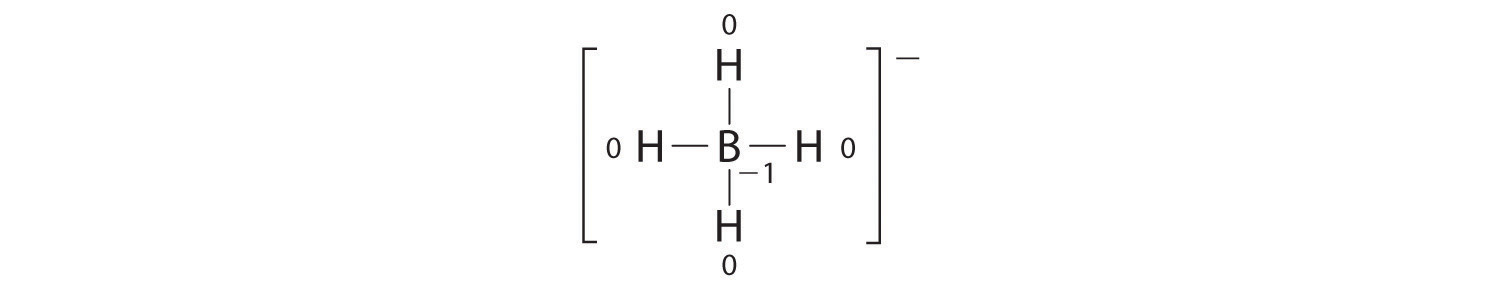
If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero.
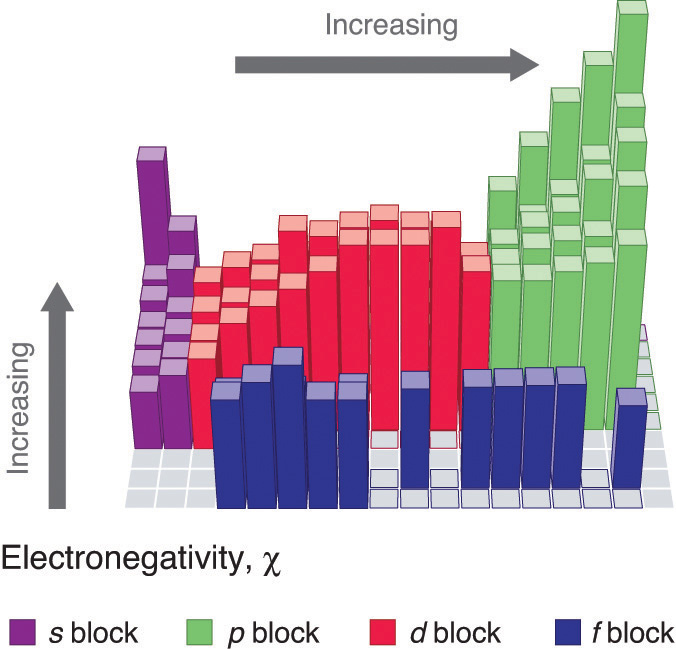
Electronegativity
The elements with the highest ionization energies are generally those with the most negative electron affinities, which are located toward the upper right corner of the periodic table. Conversely, the elements with the lowest ionization energies are generally those with the least negative electron affinities and are located in the lower left corner of the periodic table.
Because the tendency of an element to gain or lose electrons is so important in determining its chemistry, various methods have been developed to quantitatively describe this tendency. The most important method uses a measurement called electronegativity (represented by the Greek letter chi, χ, pronounced “ky” as in “sky”), defined as the relative ability of an atom to attract electrons to itself in a chemical compound. Elements with high electronegativities tend to acquire electrons in chemical reactions and are found in the upper right corner of the periodic table. Elements with low electronegativities tend to lose electrons in chemical reactions and are found in the lower left corner of the periodic table.
Unlike ionization energy or electron affinity, the electronegativity of an atom is not a simple, fixed property that can be directly measured in a single experiment. In fact, an atom’s electronegativity should depend to some extent on its chemical environment because the properties of an atom are influenced by its neighbors in a chemical compound. Nevertheless, when different methods for measuring the electronegativity of an atom are compared, they all tend to assign similar relative values to a given element. For example, all scales predict that fluorine has the highest electronegativity and cesium the lowest of the stable elements, which suggests that all the methods are measuring the same fundamental property.
Note
Electronegativity is defined as the ability of an atom in a particular molecule to attract electrons to itself. The greater the value, the greater the attractiveness for electrons.
Molecular Dipole Moments
You previously learned how to calculate the dipole moments of simple diatomic molecules. In more complex molecules with polar covalent bonds, the three-dimensional geometry and the compound’s symmetry determine whether there is a net dipole moment. Mathematically, dipole moments are vectors; they possess both a magnitude and a direction. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. If the individual bond dipole moments cancel one another, there is no net dipole moment. Such is the case for CO2, a linear molecule (part (a) in Figure 2.1.1). Each C–O bond in CO2 is polar, yet experiments show that the CO2 molecule has no dipole moment. Because the two C–O bond dipoles in CO2 are equal in magnitude and oriented at 180° to each other, they cancel. As a result, the CO2 molecule has no net dipole moment even though it has a substantial separation of charge. In contrast, the H2O molecule is not linear (part (b) in Figure 2.1.1); it is bent in three-dimensional space, so the dipole moments do not cancel each other. Thus a molecule such as H2O has a net dipole moment. We expect the concentration of negative charge to be on the oxygen, the more electronegative atom, and positive charge on the two hydrogens. This charge polarization allows H2O to hydrogen-bond to other polarized or charged species, including other water molecules.
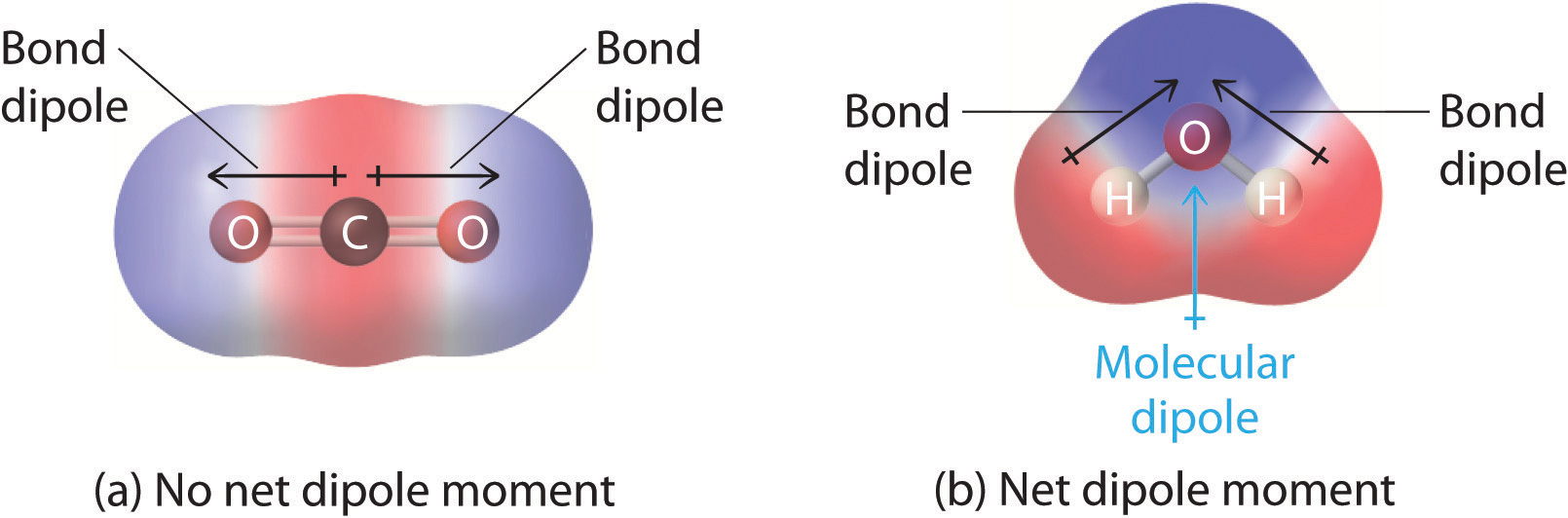
Figure 2.1.1 How Individual Bond Dipole Moments Are Added Together to Give an Overall Molecular Dipole Moment for Two Triatomic Molecules with Different Structures. (a) In CO2, the C–O bond dipoles are equal in magnitude but oriented in opposite directions (at 180°). Their vector sum is zero, so CO2 therefore has no net dipole. (b) In H2O, the O–H bond dipoles are also equal in magnitude, but they are oriented at 104.5° to each other. Hence the vector sum is not zero, and H2O has a net dipole moment.
Other examples of molecules with polar bonds are shown in Figure 2.1.2. In molecular geometries that are highly symmetrical (most notably tetrahedral and square planar, trigonal bipyramidal, and octahedral), individual bond dipole moments completely cancel, and there is no net dipole moment. Although a molecule like CHCl3 is best described as tetrahedral, the atoms bonded to carbon are not identical. Consequently, the bond dipole moments cannot cancel one another, and the molecule has a dipole moment. Due to the arrangement of the bonds in molecules that have V-shaped, trigonal pyramidal, seesaw, T-shaped, and square pyramidal geometries, the bond dipole moments cannot cancel one another. Consequently, molecules with these geometries always have a nonzero dipole moment.
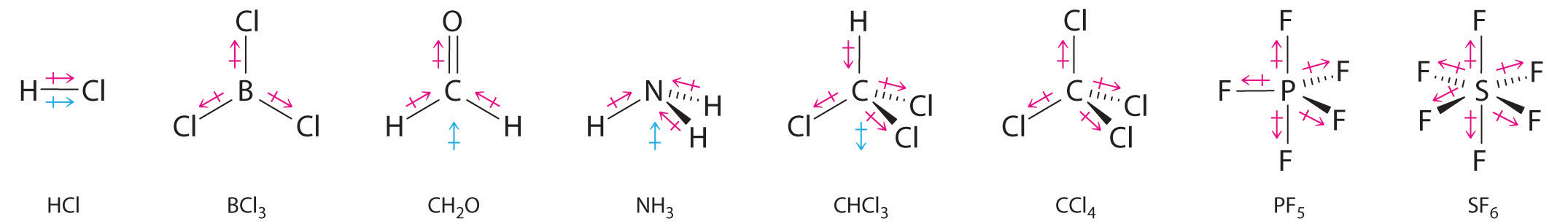
Figure 2.1.2: Molecules with Polar Bonds. Individual bond dipole moments are indicated in red. Due to their different three-dimensional structures, some molecules with polar bonds have a net dipole moment (HCl, CH2O, NH3, and CHCl3), indicated in blue, whereas others do not because the bond dipole moments cancel (BCl3, CCl4, PF5, and SF6).
Note
Molecules with asymmetrical charge distributions have a net dipole moment.
Example 2.1.2
Which molecule(s) has a net dipole moment?
- H2S
- NHF2
- BF3
Given: three chemical compounds
Asked for: net dipole moment
Strategy:
For each three-dimensional molecular geometry, predict whether the bond dipoles cancel. If they do not, then the molecule has a net dipole moment.
Solution:
-
The total number of electrons around the central atom, S, is eight, which gives four electron pairs. Two of these electron pairs are bonding pairs and two are lone pairs, so the molecular geometry of H2S is bent (Figure 9.2.6). The bond dipoles cannot cancel one another, so the molecule has a net dipole moment.
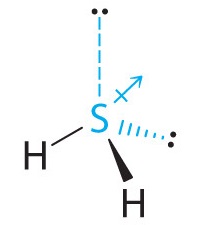
-
Difluoroamine has a trigonal pyramidal molecular geometry. Because there is one hydrogen and two fluorines, and because of the lone pair of electrons on nitrogen, the molecule is not symmetrical, and the bond dipoles of NHF2 cannot cancel one another. This means that NHF2 has a net dipole moment. We expect polarization from the two fluorine atoms, the most electronegative atoms in the periodic table, to have a greater affect on the net dipole moment than polarization from the lone pair of electrons on nitrogen.
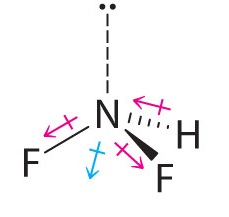
- The molecular geometry of BF3 is trigonal planar. Because all the B–F bonds are equal and the molecule is highly symmetrical, the dipoles cancel one another in three-dimensional space. Thus BF3 has a net dipole moment of zero:

Exercise 2.1.2
Which molecule(s) has a net dipole moment?
- CH3Cl
- SO3
- XeO3
Answer: CH3Cl; XeO3
In 1923, chemists Johannes Brønsted and Martin Lowry independently developed definitions of acids and bases based on compounds abilities to either donate or accept protons (H+ ions). Here, acids are defined as being able to donate protons in the form of hydrogen ions; whereas bases are defined as being able to accept protons. This took the Arrhenius definition one step further as water is no longer required to be present in the solution for acid and base reactions to occur.
Brønsted-Lowery Definition
J.N. Brønsted and T.M. Lowry independently developed the theory of proton donors and proton acceptors in acid-base reactions, coincidentally in the same region and during the same year. The Arrhenius theory where acids and bases are defined by whether the molecule contains hydrogen and hydroxide ion is too limiting. The main effect of the Brønsted-Lowry definition is to identify the proton (H+) transfer occurring in the acid-base reaction. This is best illustrated in the following equation:
| | | |
| | | |
| | | H3O+ + Cl- |
| | | NH4+ + OH- |
The determination of a substance as a Brønsted-Lowery acid or base can only be done by observing the reaction. In the case of the HOH it is a base in the first case and an acid in the second case.
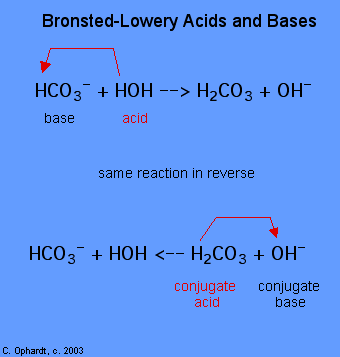
To determine whether a substance is an acid or a base, count the hydrogens on each substance before and after the reaction. If the number of hydrogens has decreased that substance is the acid (donates hydrogen ions). If the number of hydrogens has increased that substance is the base (accepts hydrogen ions). These definitions are normally applied to the reactants on the left. If the reaction is viewed in reverse a new acid and base can be identified. The substances on the right side of the equation are called conjugate acid and conjugate base compared to those on the left. Also note that the original acid turns in the conjugate base after the reaction is over.
Acids are Proton Donors and Bases are Proton Acceptors
For a reaction to be in equilibrium a transfer of electrons needs to occur. The acid will give an electron away and the base will receive the electron. Acids and Bases that work together in this fashion are called a conjugate pair made up of conjugate acids and conjugate bases.
A stands for an Acidic compound and Z stands for a Basic compound
- A Donates H to form HZ+.
- Z Accepts H from A which forms HZ+
- A- becomes conjugate base of HA and in the reverse reaction it accepts a H from HZ to recreate HA in order to remain in equilibrium
- HZ+ becomes a conjugate acid of Z and in the reverse reaction it donates a H to A- recreating Z in order to remain in equilibrium
Questions
- Why is
HA an Acid? - Why is
Z− a Base? - How can A- be a base when HA was and Acid?
- How can HZ+ be an acid when Z used to be a Base?
- Now that we understand the concept, let's look at an an example with actual compounds!
HCl+H2O⇌H3O++Cl¯
- HCL is the acid because it is donating a proton to H2O
- H2O is the base because H2O is accepting a proton from HCL
- H3O+ is the conjugate acid because it is donating an acid to CL turn into it's conjugate acid H2O
- Cl¯ is the conjugate base because it accepts an H from H3O to return to it's conjugate acid HCl
How can H2O be a base? I thought it was neutral?
Answers
- It has a proton that can be transferred
- It receives a proton from HA
- A- is a conjugate base because it is in need of a H in order to remain in equilibrium and return to HA
- HZ+ is a conjugate acid because it needs to donate or give away its proton in order to return to it's previous state of Z
- In the Brønsted-Lowry Theory what makes a compound an element or a base is whether or not it donates or accepts protons. If the H2O was in a different problem and was instead donating an H rather than accepting an H it would be an acid!
Conjugate Acid–Base Pairs
We discussed the concept of conjugate acid–base pairs in Chapter 4, using the reaction of ammonia, the base, with water, the acid, as an example. In aqueous solutions, acids and bases can be defined in terms of the transfer of a proton from an acid to a base. Thus for every acidic species in an aqueous solution, there exists a species derived from the acid by the loss of a proton. These two species that differ by only a proton constitute a conjugate acid–base pair. For example, in the reaction of
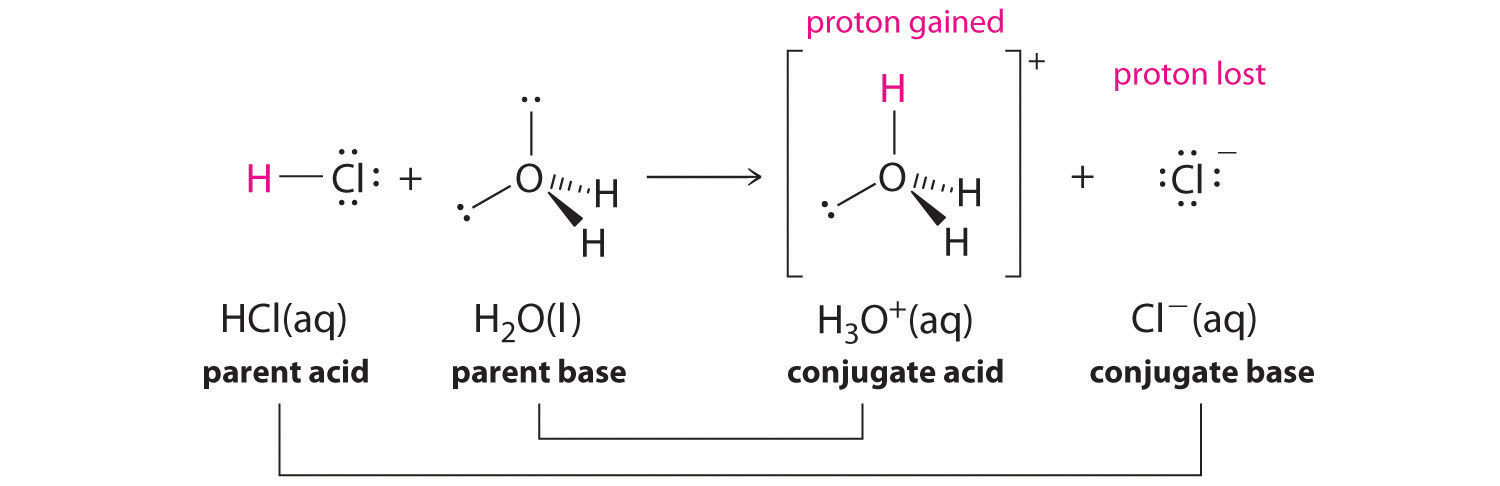
Note
All acid–base reactions contain two conjugate acid–base pairs.
Similarly, in the reaction of acetic acid with water, acetic acid donates a proton to water, which acts as the base. In the reverse reaction,
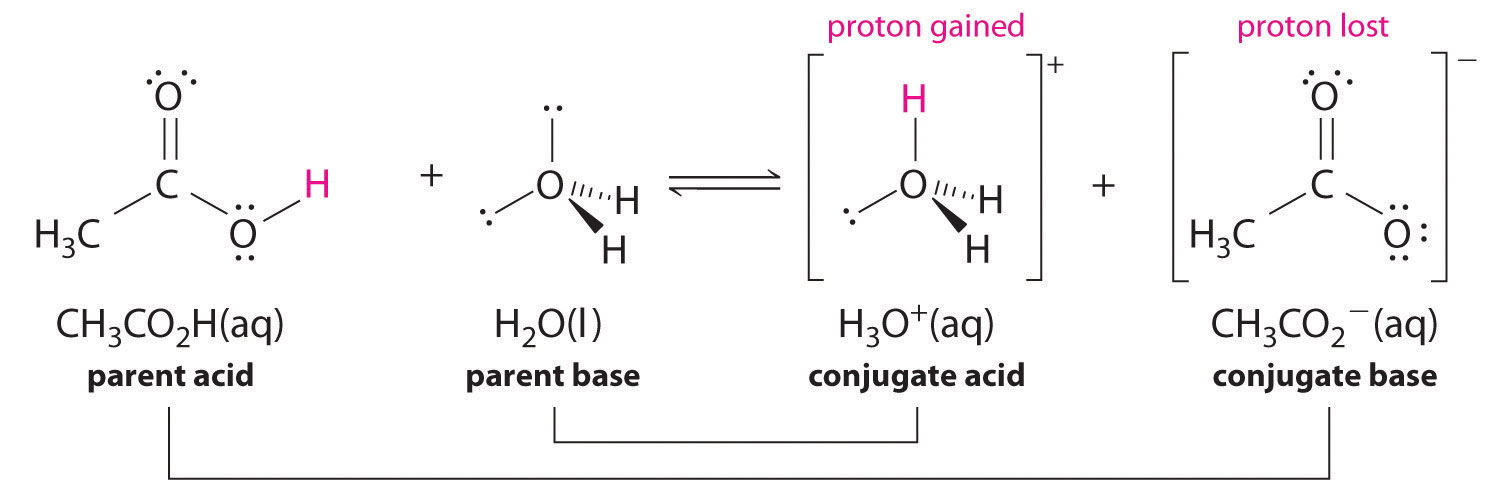
In the reaction of ammonia with water to give ammonium ions and hydroxide ions (Equation 16.3), ammonia acts as a base by accepting a proton from a water molecule, which in this case means that water is acting as an acid. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base. The conjugate acid–base pairs for this reaction are
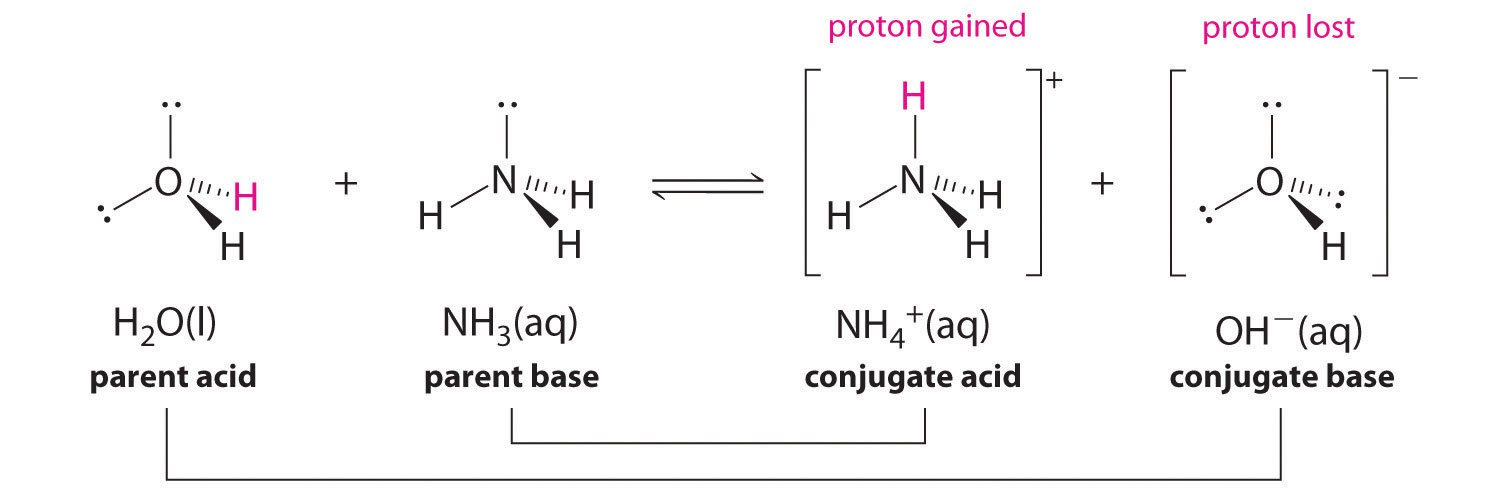
Figure 16.2 The Relative Strengths of Some Common Conjugate Acid–Base Pairs

The strongest acids are at the bottom left, and the strongest bases are at the top right. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid.
Problems
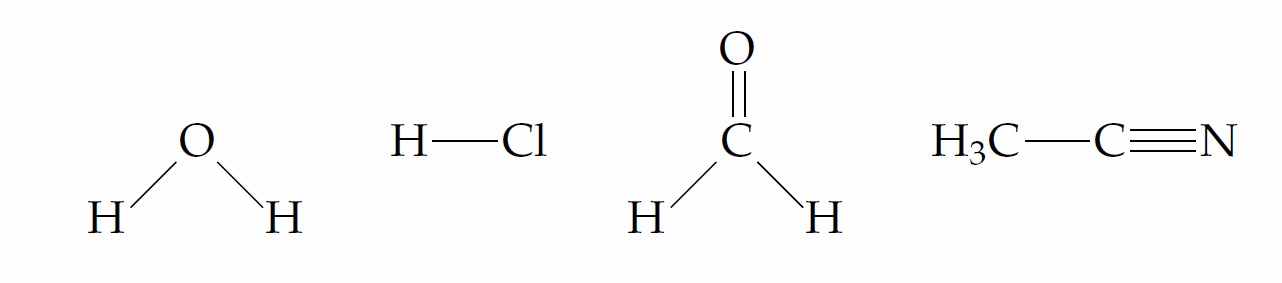
1. Identify the positive and negative ends of each of the bonds shown below.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

