8.10: Conjugation, Color, and the Chemistry of Vision (reference only)
- Page ID
- 447893
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- explain why some organic compounds have different colors based on compound structure and our perception of light.
- state the relationship between frequency of light absorbed and the extent of conjugation in an extended pi electron system.
Introduction
An obvious difference between certain compounds is their color. Thus, quinone is yellow; chlorophyll is green; and aspirin is colorless. In this respect the human eye is functioning as a spectrometer analyzing the light reflected from the surface of a solid or passing through a liquid. Although we see sunlight (or white light) as uniform or homogeneous in color, it is actually composed of a broad range of radiation wavelengths in the ultraviolet (UV), visible and infrared (IR) portions of the spectrum. As shown on the image below, the component colors of the visible portion can be separated by passing sunlight through a prism, which acts to bend the light in differing degrees according to wavelength.
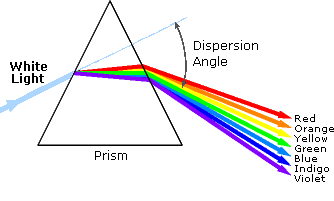
Visible wavelengths cover a range from approximately 400 to 800 nm. The longest visible wavelength is red and the shortest is violet. The wavelengths of what we perceive as particular colors in the visible portion of the spectrum are displayed and listed below.
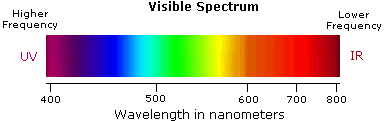
- Violet: 400 - 420 nm
- Indigo: 420 - 440 nm
- Blue: 440 - 490 nm
- Green: 490 - 570 nm
- Yellow: 570 - 585 nm
- Orange: 585 - 620 nm
- Red: 620 - 780 nm
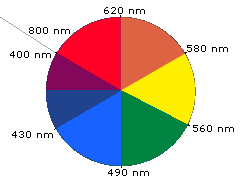
When white light passes through or is reflected by a colored substance, a characteristic portion of the mixed wavelengths is absorbed. The remaining light will then assume the complementary color to the wavelength(s) absorbed. This relationship is demonstrated by the color wheel shown below. Here, complementary colors are diametrically opposite each other. Thus, absorption of violet (400-440 nm) light renders a substance yellow, and absorption of 490-560 nm (green) light makes it red. Green is unique in that it can be created by absorption close to 400 nm as well as absorption near 800 nm.
Early humans valued colored pigments, and used them for decorative purposes. Many of these were inorganic minerals, but several important organic dyes were also known. These included the crimson pigment, kermesic acid, the blue dye, indigo, and the yellow saffron pigment, crocetin. A rare dibromo-indigo derivative, punicin, was used to color the robes of the royal and wealthy. A common feature of all these colored compounds, displayed below, is a system of extensively conjugated \(\pi\)-electrons.
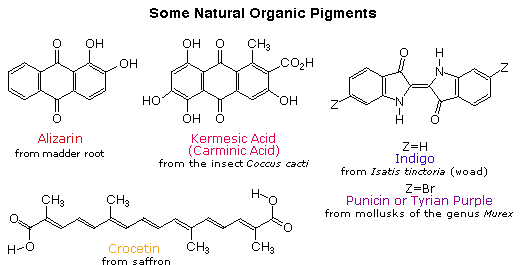
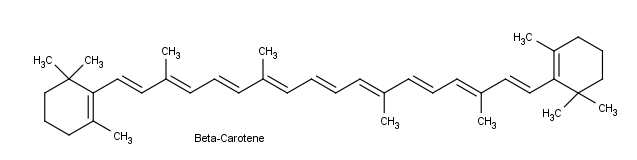
By understanding visible light, complementary colors, and adsorption the color of organic compounds can be understood. Beta-carotene, a compound found in carrots, is a deep orange color. Beta-carotene has 11 conjugated double bonds which places its λmax at 455 nm which is within the blue region of the visible spectrum. The Beta-carotene compound absorbs blue from white light so it appears orange which is the the complementary color of blue.
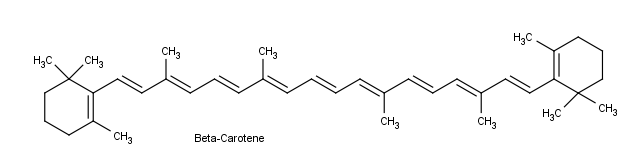
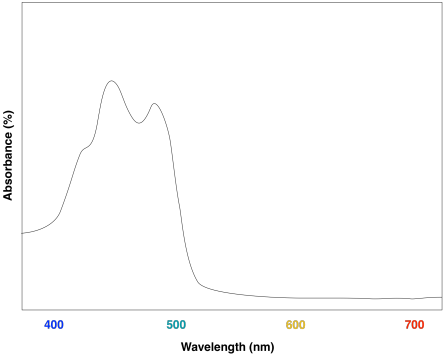
Another example is seen in the absorption spectrum of another food coloring, Blue #1:
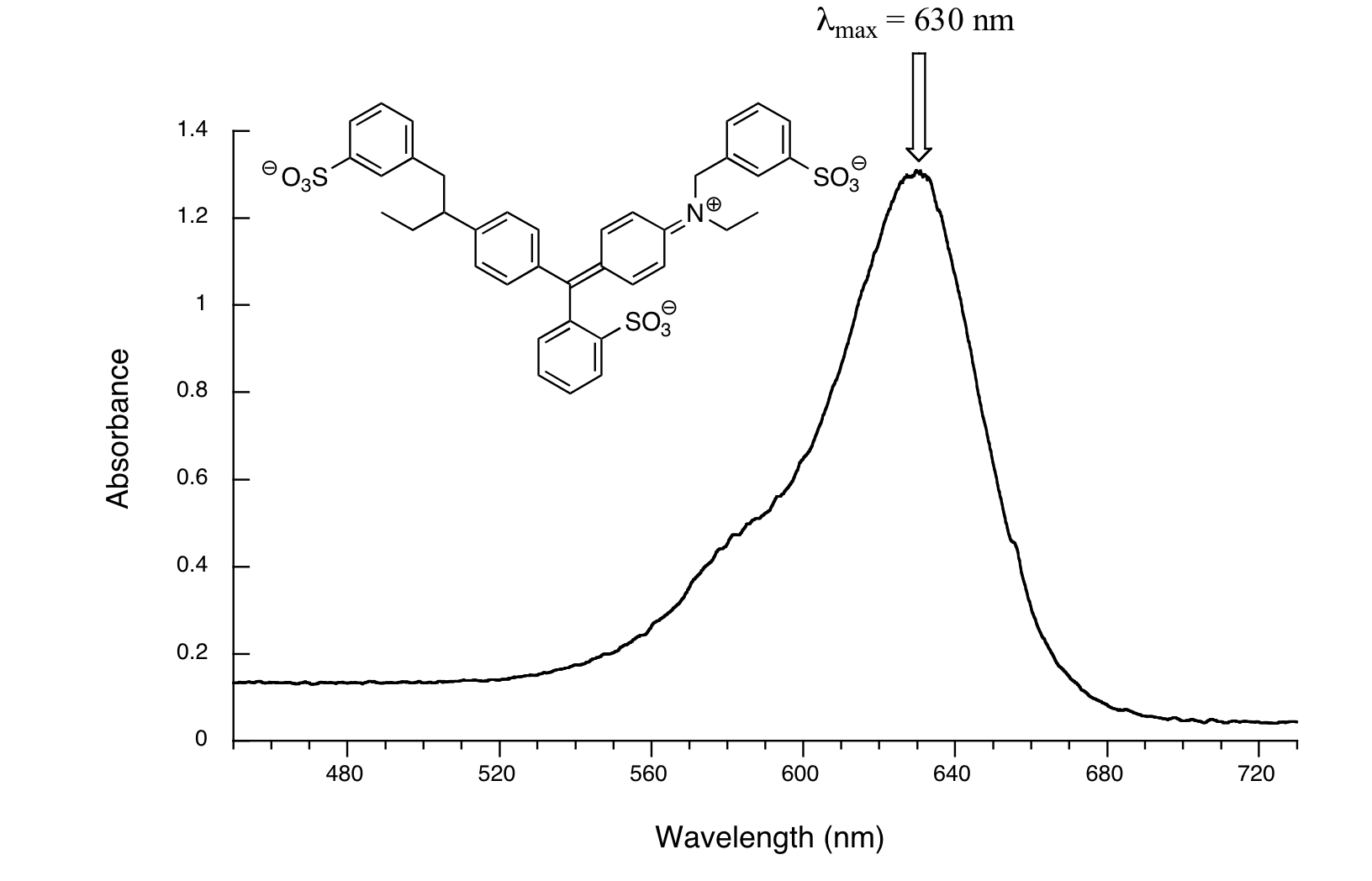
Blue #1 absorbs at a λmax at 630 nm which is the color red in the visible spectrum. Blue is the complementary color of red.
Mechanism of Vision
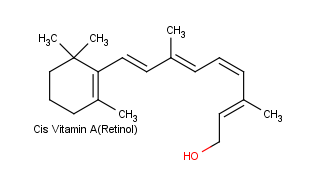
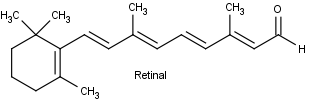
Conjugation is also important in light sensitive compounds used for vision. Beta carotene, found in carrots and other natural products is cleaved into the liver and converted into Vitamin A, also known as retinol. Vitamin A is critical for vision because it is needed by the retina of eye. Retinol can be oxidized to an aldehyde called retinal which is also important for vision.
The eye is an extraordinarily sensitive instrument. Although its wavelength response is restricted to 400-800 nm, but its degree of sensitivity is such that a fully dark-adapted eye can clearly detect objects in light so dim as to correspond to a light input over the retina of only about 10,000 quanta per second - one light quantum per three minutes per receptor cell in the retina!
The retina is made up of two kinds of light-sensitive (photoreceptor) cells, known as rods and cones. The rods are the more sensitive and are responsible for vision in dim light. The cones are much fewer in number than the rods and provide detail and color vision in good light. The part of the retina that corresponds to the center of the visual field contains only cones. A red pigment called rhodopsin is the photosensitive substance in the rod cells of the retina. It absorbs most strongly in the blue-green region of the visible spectrum (λmax = 500nm) and is essentially unaffected by the far-red end of the spectrum. Cone vision appears to involve a different pigment called iodopsin, which absorbs farther toward the red than does rhodopsin.
Rhodopsin, is made up of a protein (opsin) and retinal. Opsin does not absorb visible light, but when it is bonded with 11-cis-retinal to from rhodopsin, the new molecule has a very broad absorption band in the visible region of the spectrum. Rhodopsin is formed by an an imine (Schiff base) functional group formed between the aldehyde group of the retinal and the side-chain amino function of a lysine unit of opsin.
Opsin itself is colorless, whereas 11-cis-retinal absorbs strongly at 370 nm. The combination of opsin with 11-cis-retinal produces a remarkable shift of λmax to longer wavelengths (430 nm to 620 nm, depending on the species). Light striking the retina changes the color of rhodopsin from red to yellow. The primary photochemical event in this process was established by G. Wald (Nobel Laureate in Physiology and Medicine, 1967), who showed that light absorption led to a change of configuration about the C11-C12 double bond of the retinal moiety fo rhodopsin from cis to trans to form a compound called metarhodopsin II. The new form of trans-retinal does not fit as well into the opsin protein, and so a series of geometry changes in the protein begins. As the protein changes its geometry, it initiates a cascade of biochemical reactions that result in changes in charge so that a large potential difference builds up across the neuron membranes. This potential difference is passed along to an adjoining nerve cell as an electrical impulse. The nerve cell carries this impulse to the brain, where the visual information is interpreted.
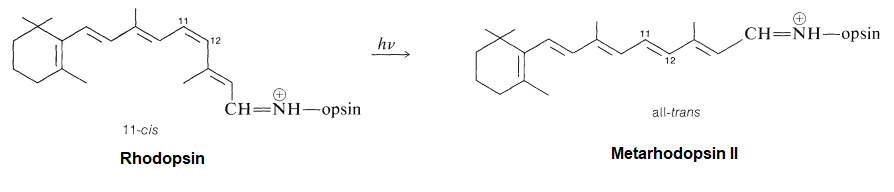
Metarhodopsin II can then be recycled back to rhodopsin by first cleaving to form all-trans-retinal and the isomerization back to 11-cis-retinal by the enzyme retinal isomerase. Finally, 11-cis-retinal is once again coupled with opsin to form rhodopsin.

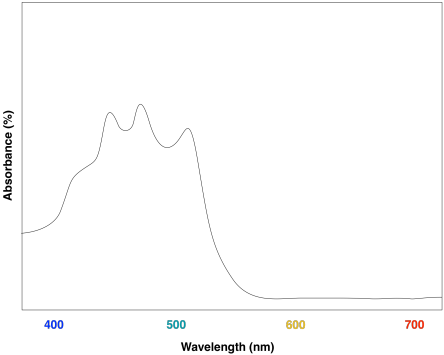
Lycopene is conjugated compound found in tomatoes. What colors correspond to the UV-Vis absorption maxima of lycopene? What color do we see when we look at lycopene?
- Answer
-
The absorption maxima of lycopene are regions of blue and green in the visible spectrum. Lycopene would be expected to appears orange and red.
Asprin has a λmax of 220 nm. Briefly explain why Asprin appears white.
- Answer
-
Asprin absorbs in the ultraviolet portion of the electromagnetic spectrum. Asprin cannot remove any colors from white light by absorption so the compound itself appears white. Many compounds with a relatively small amount of conjugation appear white because they only absorb in the ultraviolet and not the visible region.

