3.7: Axial and Equatorial Bonds in Cyclohexane
- Page ID
- 178977
Objectives
After completing this section, you should be able to
- Draw the chair conformation of cyclohexane, with axial and equatorial hydrogen atoms clearly shown and identified.
- identify the axial and equatorial hydrogens in a given sketch of the cyclohexane molecule.
- explain how chair conformations of cyclohexane and its derivatives can interconvert through the process of ring flip.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- axial position
- equatorial position
- ring flip
Cyclohexane Conformations (aka Chair Flips)
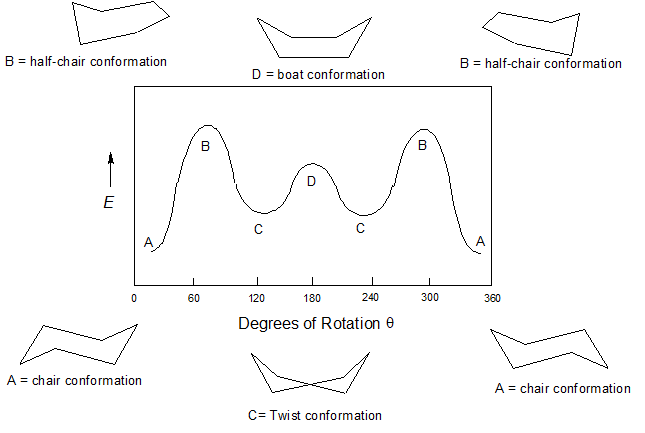
Cyclohexane is rapidly rotating between the two most stable conformations known as the chair conformations in what is called the "Chair Flip" shown below.

Several other notable cyclohexane conformations occur during the transition from one chair conformer to the other - the boat, the twist, and the half-chair. The relative energies of the conformations is a direct reflection of their relative stabilities. These structural and energetic relationships are summarized in the conformational energy diagram for cyclohexane below.
The Chair Conformation - a closer look
Careful examination of the chair conformation of cyclohexane, shows that the twelve hydrogens are not structurally equivalent. Six of them are located about the periphery of the carbon ring, and are termed equatorial. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring.
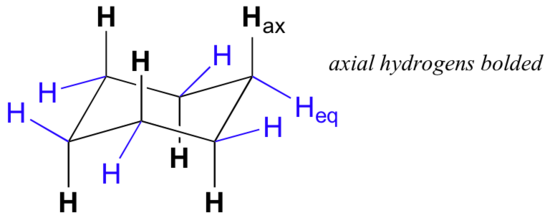
In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are black. Since there are two equivalent chair conformations of cyclohexane in rapid equilibrium, all twelve hydrogens have 50% equatorial and 50% axial character. The figure below illustrates how to convert a molecular model of cyclohexane between two different chair conformations - this is something that you should practice with models. Notice that a 'ring flip' causes equatorial hydrogens to become axial, and vice-versa.
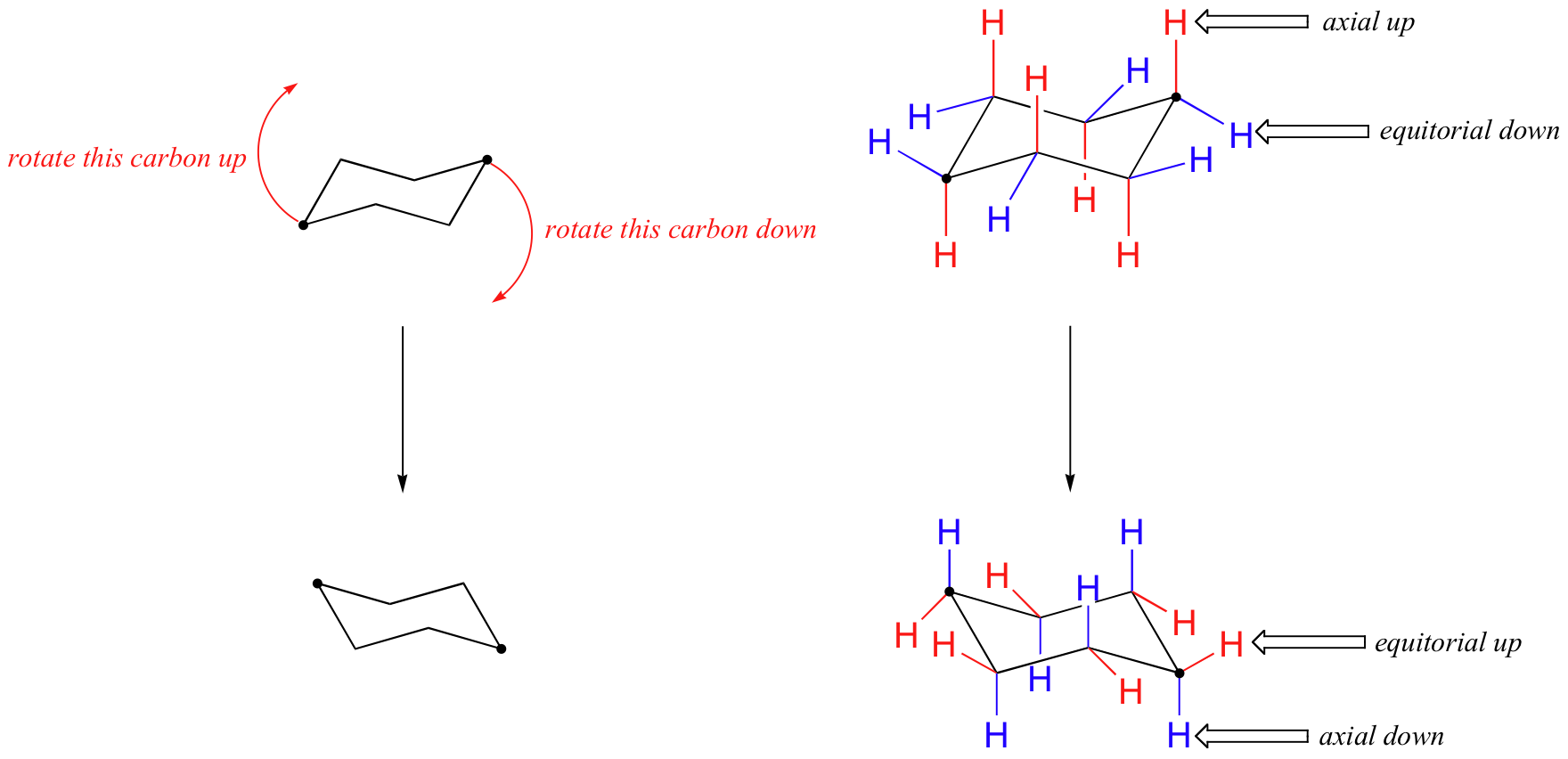
How to Draw chairs
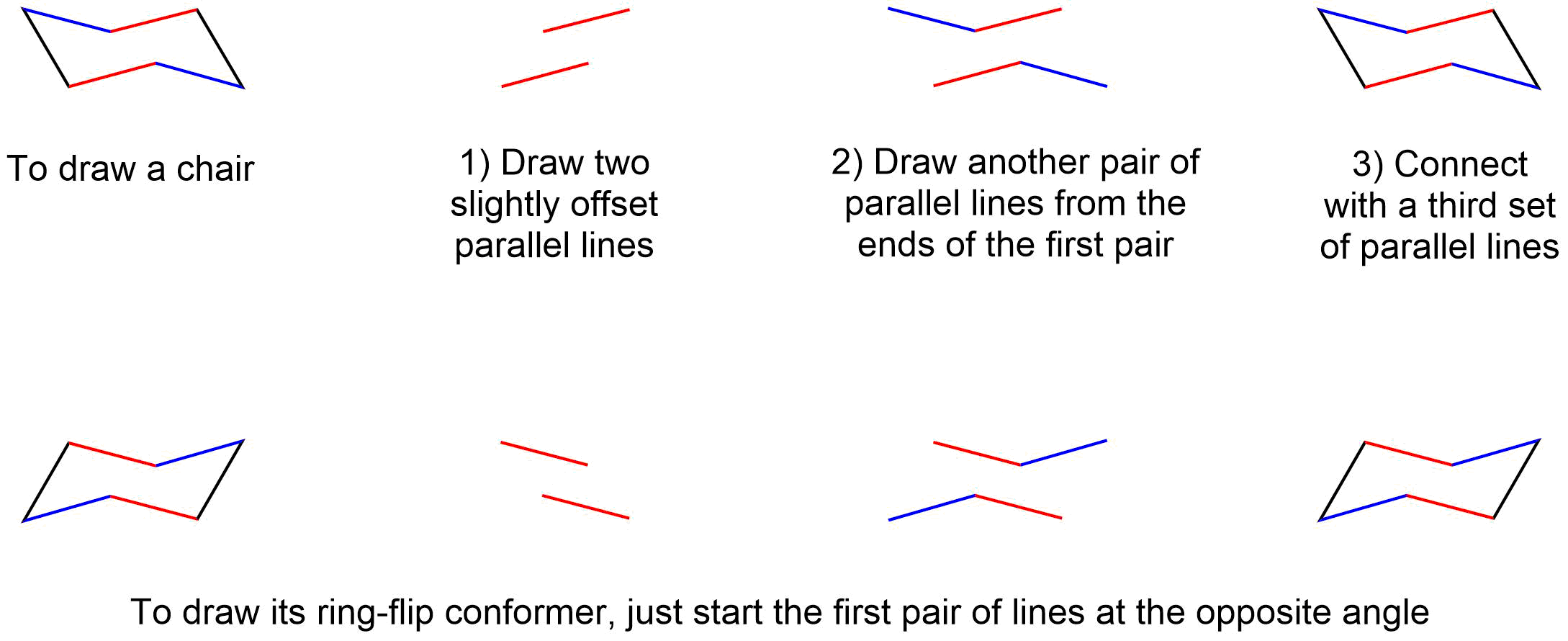
To draw the axial and equatorial bonds
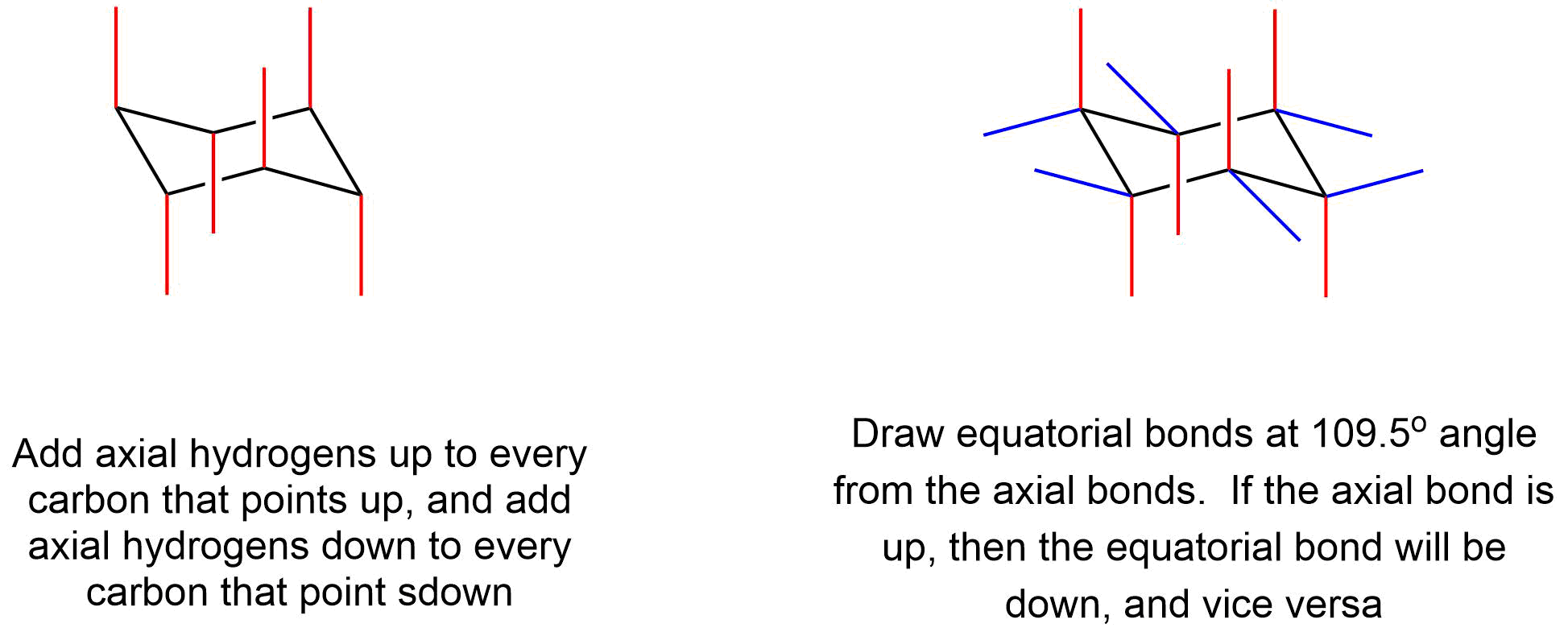
Aside from drawing the basic chair, the key points are:
- Axial bonds alternate up and down, and are shown "vertical".
- Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109.5o bond angle.
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Dr. Kelly Matthews, Harrisburg Area Community College

