3.9: Conformations of Disubstituted Cyclohexanes
- Page ID
- 178979
Objective
After completing this section, you should be able to use conformational analysis to determine the most stable conformation of a given disubstituted cyclohexane.
Key Terms
Make certain that you can define, and use in context, the key term below.
- conformational analysis
Study Notes
When faced with the problem of trying to decide which of two conformers of a given disubstituted cyclohexane is the more stable, you may find the following generalizations helpful.
- A conformation in which both substituents are equatorial will always be more stable than a conformation with both groups axial.
-
When one substituent is axial and the other is equatorial, the most stable conformation will be the one with the bulkiest substituent in the equatorial position. Steric bulk decreases in the order
tert-butyl > isopropyl > ethyl > methyl > hydroxyl > halogens
Monosubstituted Cyclohexanes
In the previous section, it was stated that the conformation in which the methyl group is equatorial is more stable because it minimizes steric repulsion, and thus the equilibrium favors the more stable conformer.
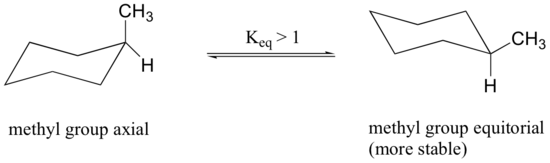
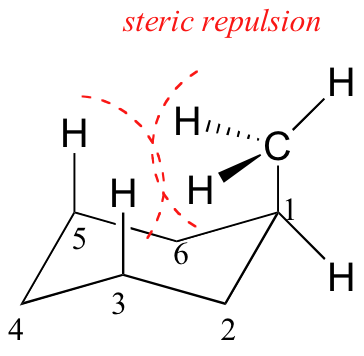
Disubstituted Cyclohexanes
In this section, the effect of conformations on the relative stability of disubstituted cyclohexanes is examined using the two principles:
- Chair conformations are generally more stable than other possibilities, so that's all we'll consider.
- Substituents prefer equatorial rather than axial positions in order to minimize the steric hindrance of 1,3-diaxial interactions.
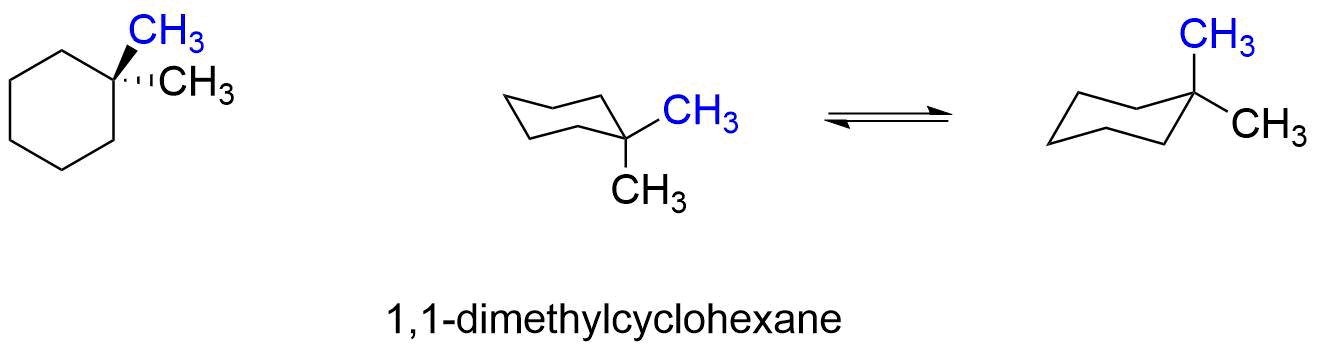
1,1-dimethylcyclohexane does not have cis or trans isomers, because both methyl groups are on the same ring carbon. Both conformers have one methyl group axial and one methyl group equatorial, and they both have the same energy or relative stability. Therefore, the equilibrium between the two conformers does not favor either one. The energy cost of having one methyl group axial (versus equatorial) is 1.7 kcal/mole (from Table 4.7.1), and both conformers will be equal in energy. Note that both methyl groups cannot be equatorial at the same time without breaking bonds and creating a different molecule.
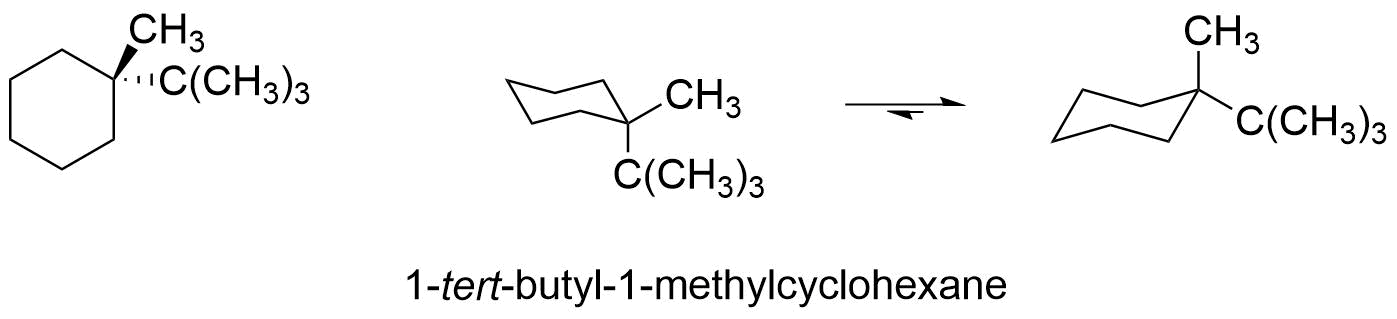
However, if the two groups are different, as in 1-tert-butyl-1-methylcyclohexane, then the equilibrium favors the conformer in which the larger group tert-butyl group is in the more stable equatorial position. The energy cost of having one tert-butyl group axial (versus equatorial) can be calculated from the values in table 4.7.1 and is approximately 5.0 kcal/mole. That means that the conformer with the tert-butyl group axial is approximately 3.3 kcal/mol (5.0 kcal/mol - 1.7 kcal.mole) less stable then the conformer with the tert-butyl group equatorial. This means that 1-tert-butyl-1-methylcyclohexane will spend the majority of its time in the more stable conformation.
Disubstituted Cyclohexanes: The Relative Stability of cis and trans Isomers
The situation is more complex when the effect of conformations on the relative stability of cis and trans disubstituted cyclohexanes is analyzed. Remember, configurational stereoisomers do not interconvert without breaking bonds, whereas conformational isomers normally interconvert rapidly by the ring flip process.
Let's apply a similar analysis to the cis and trans stereoisomers of 1,2-dimethylcyclohexane.
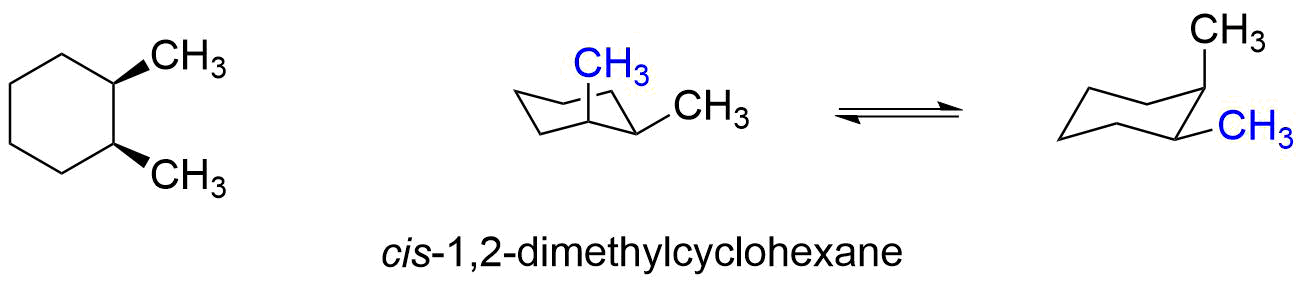
In cis-1,2-dimethylcyclohexane, one methyl group is axial and one methyl group is equatorial in both ring flip conformers, so neither conformer is more stable than the other. It is important to note, that this molecule cannot get both methyl groups axial without breaking bonds to make a new molecule.
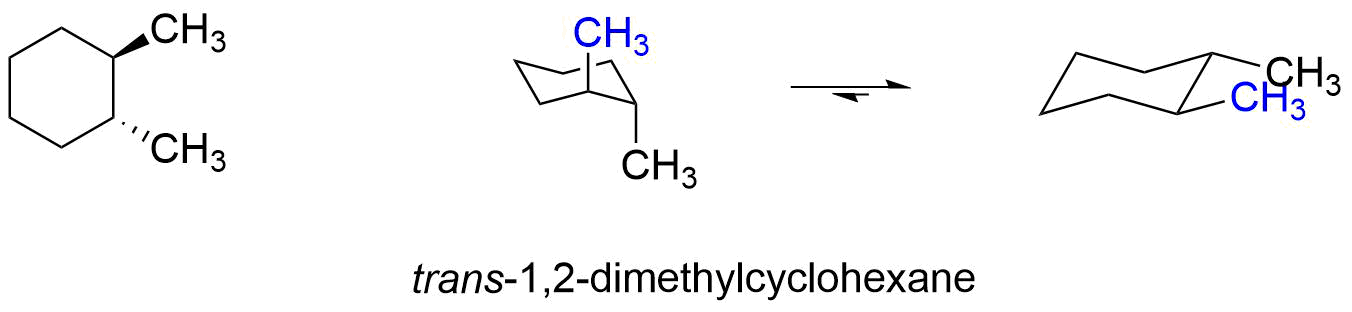
In trans-1,2-dimethylcyclohexane, one conformer has both methyl groups axial and the other has both methyl groups equatorial. The conformer with both methyl groups axial is 3.4 kcal/mole (2 x 1.7 kcal/mol) less stable than the conformer with both methyl groups equatorial. Obviously, the equilibrium will favor the conformer with both methyl groups in the equatorial position.
To decide whether the cis or trans isomer of 1,2-dimethylcyclohexane is more stable, compare the relative energy of the most stable conformer of cis-1,2-dimethylcyclohexane to the most stable conformer of trans-1,2-dimethylcyclohexane. The trans-1,2-dimethylcyclohexane has the most stable conformer, so it is the more stable isomer.
Does this mean that the trans isomer of a disubstituted cyclohexane is always more stable than the cis isomer? Let's examine both the cis and trans isomers of 1,3-dimethylcyclohexane and find out.
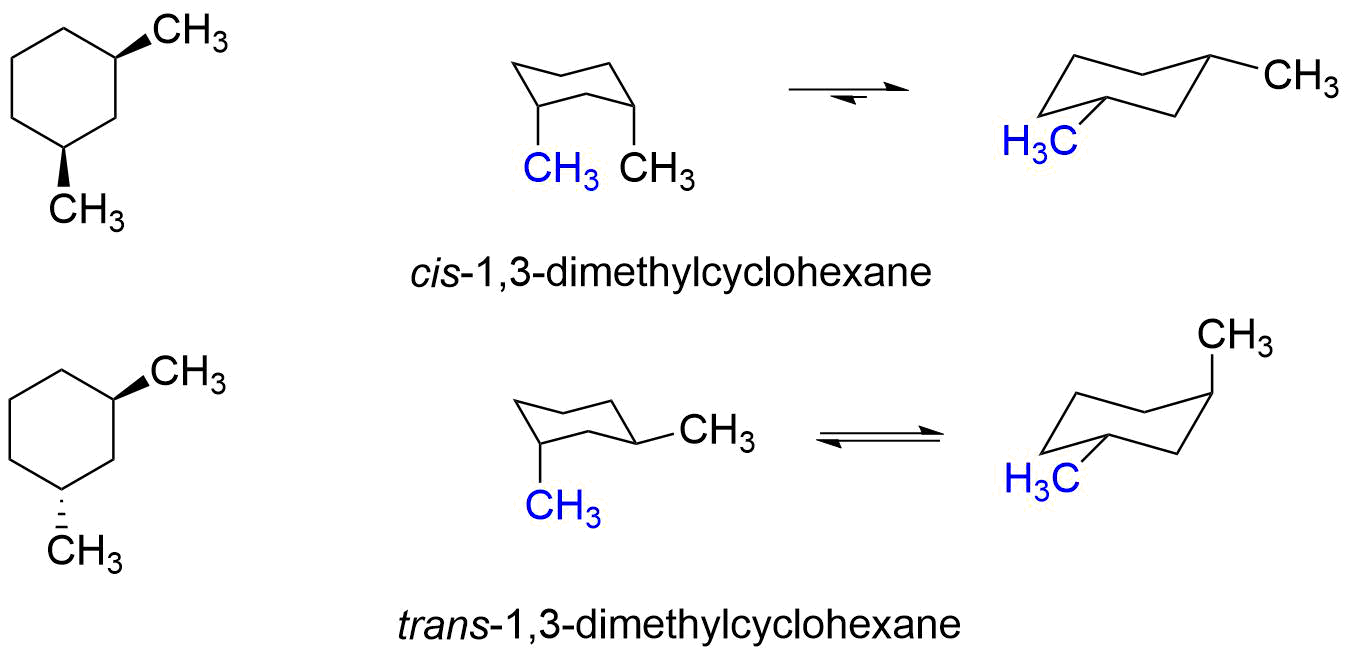
As can be seen in the structures above, cis-1,3-dimethylcyclohexane's most stable conformer has both methyl groups equatorial, while trans-1,3-dimethylcyclohexane always has one methyl group is equatorial and one methyl group axial. This means that the cis isomer 1,3-dimethylcyclohexane is more stable than the trans isomer. And that is opposite what we found for 1,2-dimethylcyclohexane.
As practice, you should do a similar conformational analysis of the cis and trans isomers of 1,4-dimethylcyclohexane. You should find that the trans isomer of 1,4-dimethylcyclohexane is more stable than the cis isomer.
Summary
The relative stabilities of the cis and trans isomers of disubstituted cyclohexanes depends upon which isomer has the most stable conformer. The most stable isomer for disubstituted cyclohexanes is summarized below.
| substitution type | most stable isomer |
| 1,2-disubstituted cyclohexanes | trans |
| 1,3-disubstituted cyclohexanes | cis |
| 1,4-disubstituted cyclohexanes | trans |
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Jim Clark (Chemguide.co.uk)
>Robert Bruner (http://bbruner.org)
- Kelly Matthews, Senior Professor of Chemistry, Harrisburg Area Community College

