1.8: Drawing Resonance Forms
- Page ID
- 178950
Objectives
After completing this section, you should be able to
- draw the resonance structures of molecules or ions that exhibit delocalization.
- determine the relative stability of resonance structures using a set of rules.
- use the concept of resonance to explain structural features of molecules and ions.
key words
- resonance structure
- resonance hybrid
Resonance Contributors for the Carboxylate Group
The convention of drawing two or more resonance contributors to approximate a single structure may seem a bit clumsy at this point, but the practice is actually very useful when discussing the manner in which many functional groups react. Let’s consider formate, the simplest carboxylate ion. The conjugate acid of formate is formic acid, which causes the painful sting you felt if you have ever been bitten by an ant.
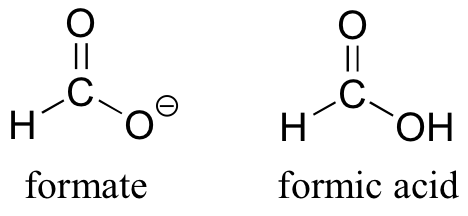
Usually, carboxylate ions are drawn with one carbon-oxygen double bond and one carbon-oxygen single bond, with a negative formal charge located on the single-bonded oxygen. However, the two carbon-oxygen bonds are actually the same length, and although there is indeed an overall negative formal charge on the group, it is shared equally between the two oxygens. Therefore, the carboxylate ion can be more accurately depicted by a pair of resonance contributors. Alternatively, a single structure can be used, with a dashed line depicting the resonance-delocalized pi bond and the negative charge located in between the two oxygens.
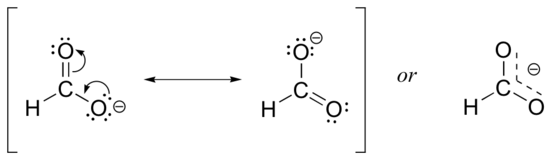
Let’s see if we can correlate these drawing conventions to a valence bond theory picture of the bonding in a carboxylate group. We know that the carbon must be sp2-hybridized, (the bond angles are close to 120˚, and the molecule is planar), and we will treat both oxygens as being sp2-hybridized as well. Both carbon-oxygen sigma bonds, then, are formed from the overlap of carbon sp2 orbitals and oxygen sp2 orbitals.
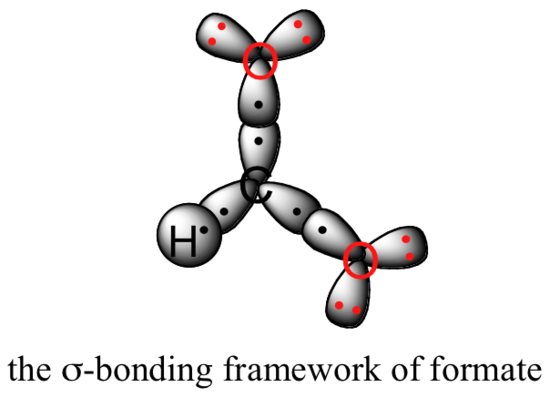
In addition, the carbon and both oxygens each have an unhybridized 2pz orbital situated perpendicular to the plane of the sigma bonds. These three 2pz orbitals are parallel to each other, and can overlap in a side-by-side fashion to form a delocalized pi bond.
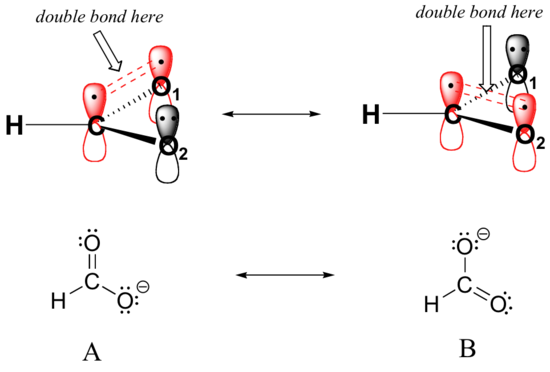
Resonance contributor A shows oxygen #1 sharing a pair of electrons with carbon in a pi bond, and oxygen #2 holding a lone pair of electrons in its 2pz orbital. Resonance contributor B, on the other hand, shows oxygen #2 participating in the pi bond with carbon, and oxygen #1 holding a lone pair in its 2pz orbital. Overall, the situation is one of three parallel, overlapping 2pz orbitals sharing four delocalized pi electrons. Because there is one more electron than there are 2pz orbitals, the system has an overall charge of –1. This is the kind of 3D picture that resonance contributors are used to approximate, and once you get some practice you should be able to quickly visualize overlapping 2pz orbitals and delocalized pi electrons whenever you see resonance structures being used. In this text, carboxylate groups will usually be drawn showing only one resonance contributor for the sake of simplicity, but you should always keep in mind that the two C-O bonds are equal, and that the negative charge is delocalized to both oxygens.
Exercise 2.6.1: There is a third resonance contributor for formate (which we will soon learn is considered a 'minor' contributor). Draw this resonance contributor.
Here's another example, this time with a carbocation. Recall that carbocations are sp2-hybridized, with an empty 2p orbital oriented perpendicular to the plane formed by three sigma bonds. If a carbocation is adjacent to a double bond, then three 2p orbitals can overlap and share the two pi electrons - another kind of conjugated pi system in which the positive charge is shared over two carbons.

Using Curved Arrows to Show Resonance
There are only three types of electron "motion" in resonance. They are
- A lone pair forms a pi bond to an adjacent atom
- A pi bond forms a new pi bond to an adjacent atom
- A pi bond forms a lone pair on adjacent atom
Let's look at the resonance within acrylic acid to demonstrate these three types of resonance.
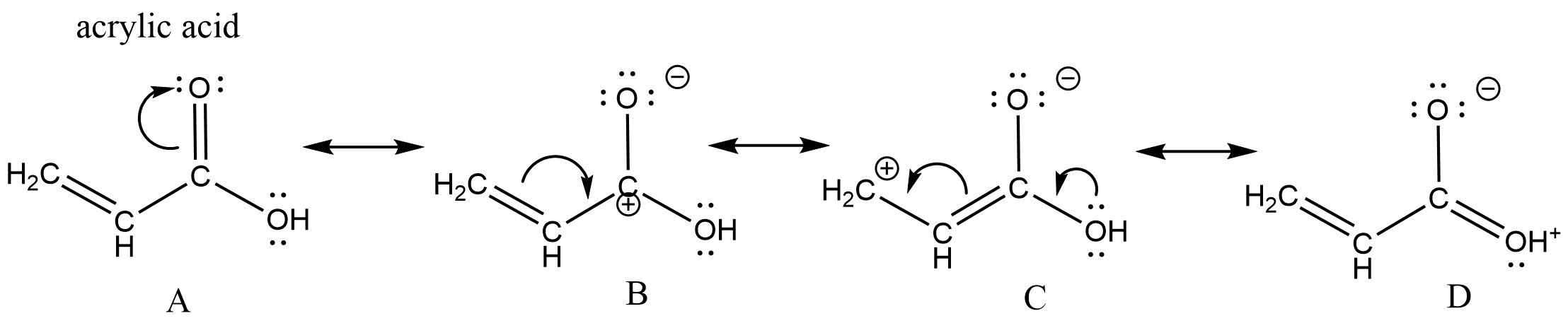
The curved arrow in structure A represents the type 3 resonance "motion" - the pi bond between the carbon and oxygen breaks to form another lone pair on the oxygen. The curved arrow in structure B represents type 2 resonance "motion" - the pi bond breaks to form a new pi bond to the carbocation carbon. In structure C, there are two curved arrows. The curved arrow from the oxygen lone pair is type 1 resonance motion - the lone pairs forms a new pi bond between the oxygen and carbon. The other arrow in structure C moves the pi bond to the end of the chain - what type of resonance is that?
Exercise 2.6.2: Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below.
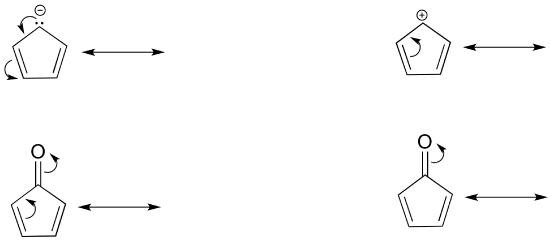
Exercise 2.6.3: In each resonance expression, identify the type of resonance motion. Then draw curved arrows on the left-side contributor that shows how we get to the right-side contributor.
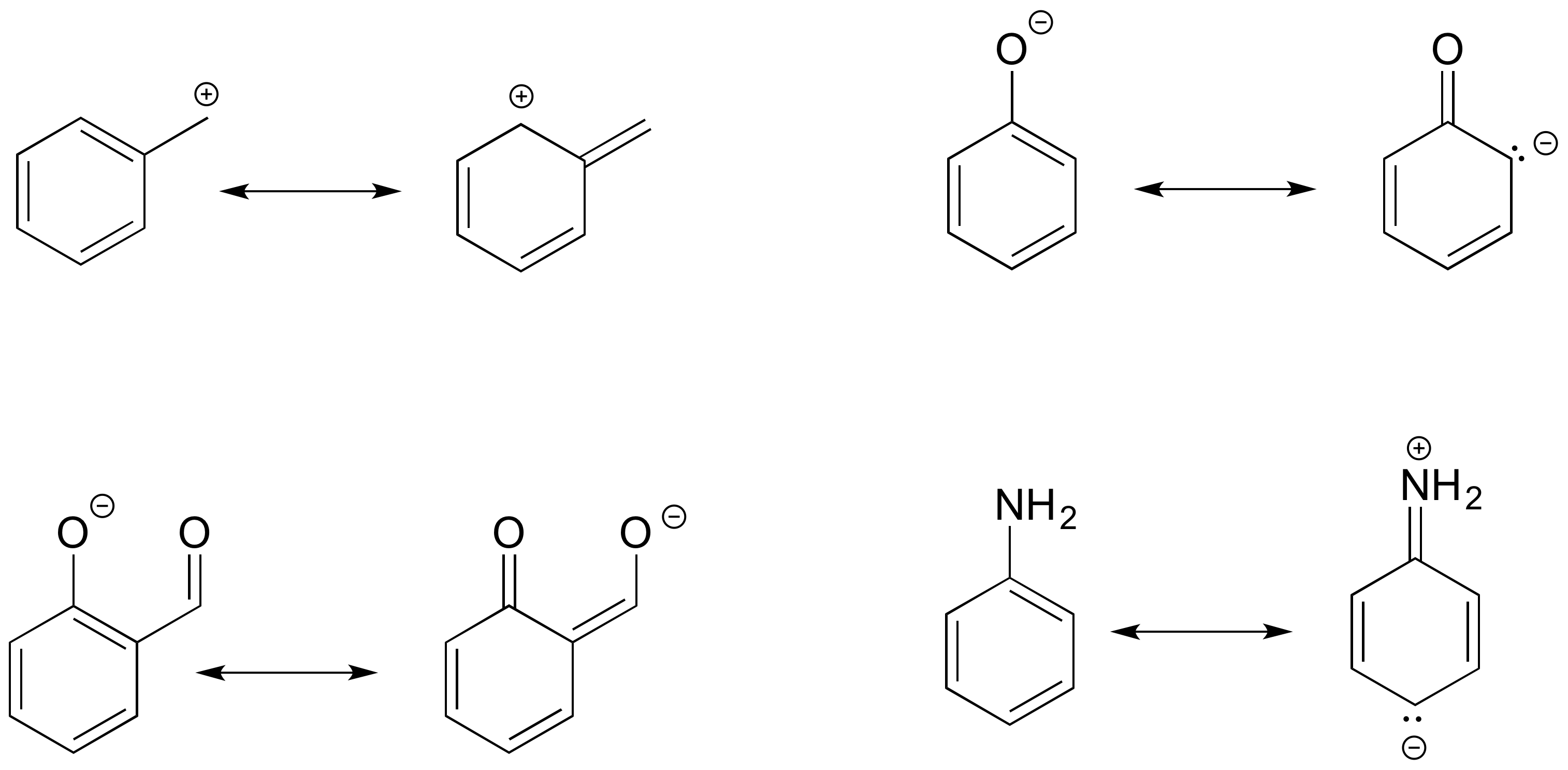
Guided Resonance Practice
Below are a few more examples of 'legal' resonance expressions. Confirm for yourself that the octet rule is not exceeded for any atoms, and that formal charges are correct.
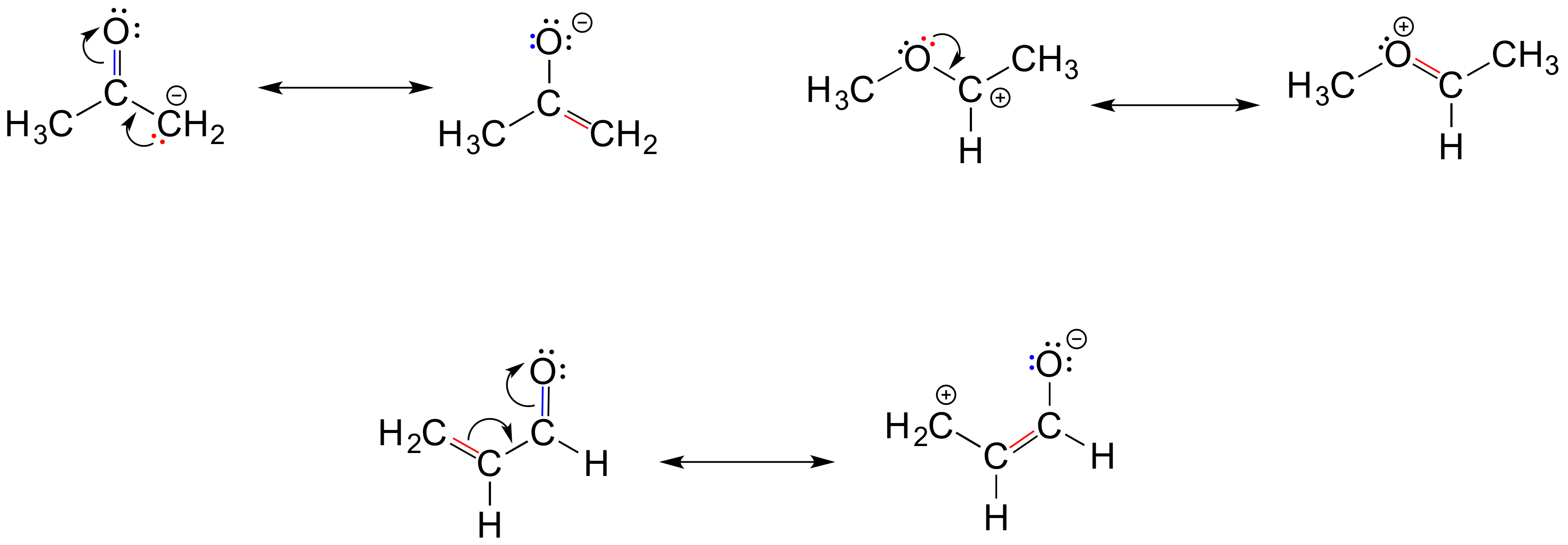
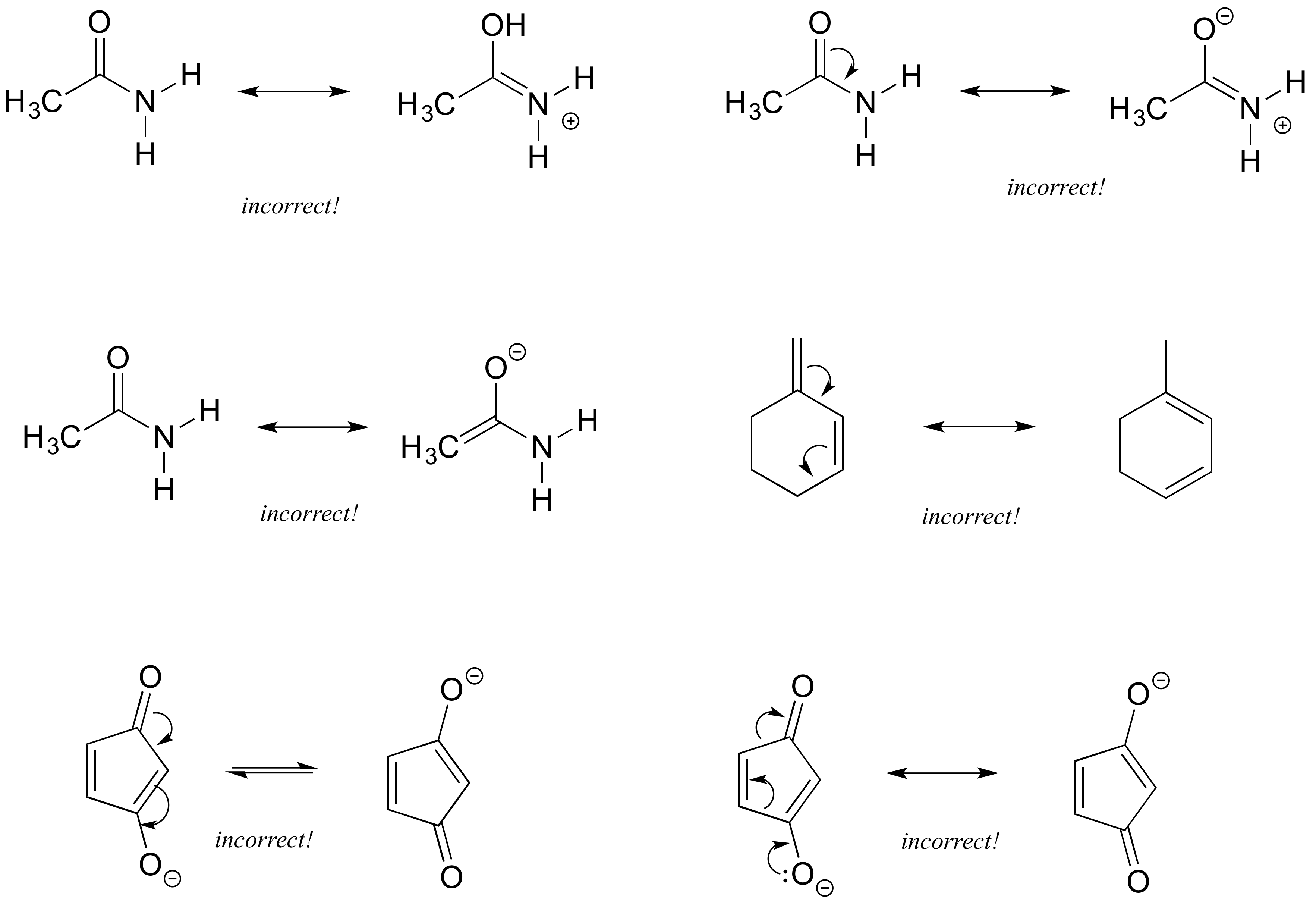
Exercise 2.6.4: Each of the 'illegal' resonance expressions below contains one or more mistakes. Explain what is incorrect in each.
Evaluating Resonance Contributors
1. Identical structures are equally important.
2. Structures will a greater number of bonds are more important.
3. Structures with charge separation are less important.
4. Pay attention to electronegativities.
5. Neutral atoms need to have complete octets
Major vs minor resonance contributors
Different resonance contributors do not always make the same contribution to the overall structure of the hybrid - rather, in many cases one contributor comes closer to depicting the actual bonding picture than another.
Rules for estimating relative stability of resonance structures
- The structure in which all atoms have a complete valence shell is more stable.
- The structure that has the greater number of covalent bonds is more stable.
- The structure with the fewest number and/or the least separation of formal charges is more stable.
- The structure with negative charge on the more electronegative atom is more stable. Positive charges should be on the less electronegative atom.
- Resonance forms that are equivalent have no difference in stability and contribute equally. (eg. benzene)
In the case of carboxylates, contributors A and B below are equivalent in terms of their relative contribution to the hybrid structure. However, there is also a third resonance contributor ‘C, in which the carbon bears a positive formal charge (a carbocation) and both oxygens are single-bonded and bear negative charges.
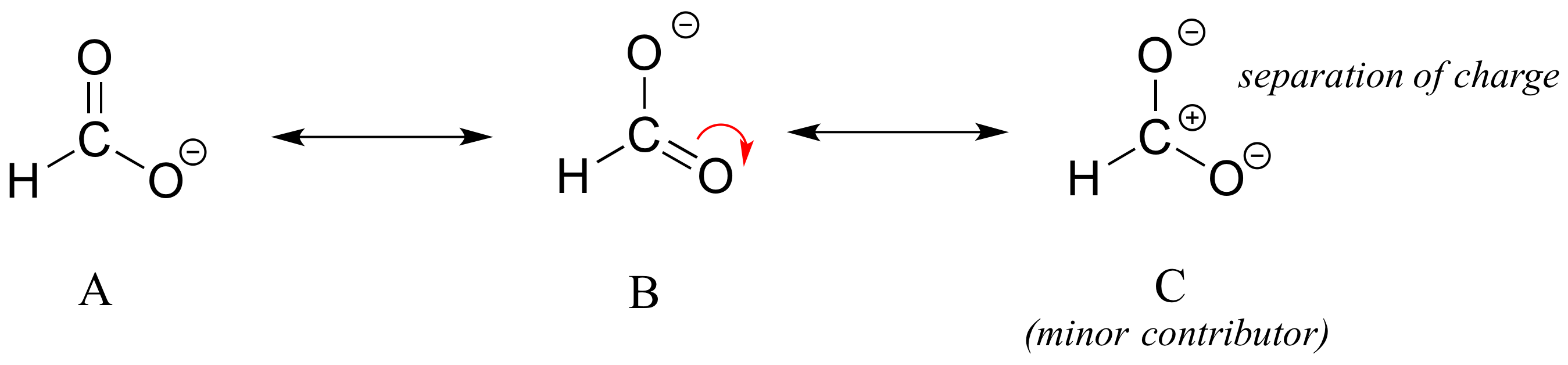
Structure C makes a less important contribution to the overall bonding picture of the group relative to A and B. How do we know that structure C is the ‘minor’ contributor? Apply the rules below
- The carbon in contributor C does not have an octect. In general, resonance contributors in which a carbon does not fulfill the octet rule are relatively less important. (rule #1)
- In structure C, there are only three bonds, compared to four in A and B. In general, a resonance structure with a lower number of total bonds is relatively less important. (rule #2)
- Structure C also has more formal charges than are present in A or B. In general, resonance contributors in which there is more/greater separation of charge are relatively less important. (rule #3)
- Structures A and B are equivalent and will be equal contributors to the resonance hybrid. (rule #5).
- The resonance contributor in which a negative formal charge is located on a more electronegative atom, usually oxygen or nitrogen, is more stable than one in which the negative charge is located on a less electronegative atom such as carbon. An example is in the upper left expression in the next figure. (rule #4)
Solved example: Draw the major resonance contributor of the structure below. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. Explain why your contributor is the major one. In what kind of orbitals are the two lone pairs on the oxygen?
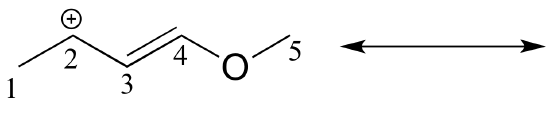
Solution: In the structure above, the carbon with the positive formal charge does not have a complete octet of valence electrons. Using the curved arrow convention, a lone pair on the oxygen can be moved to the adjacent bond to the left, and the electrons in the double bond shifted over to the left (see the rules for drawing resonance contributors to convince yourself that these are 'legal' moves).
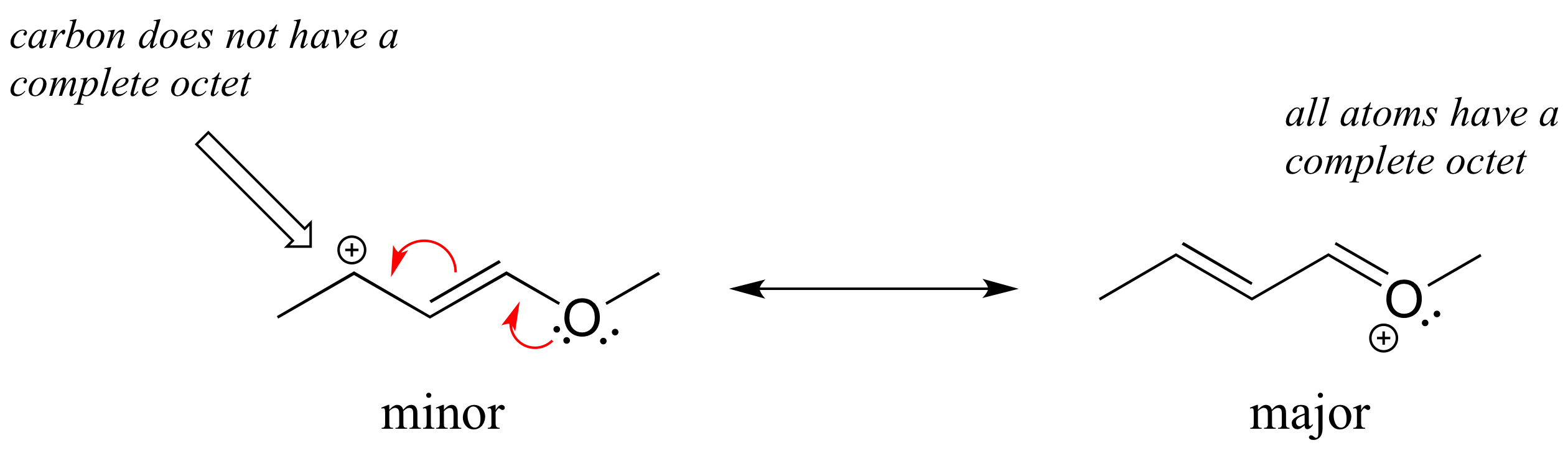
The resulting resonance contributor, in which the oxygen bears the formal charge, is the major one because all atoms have a complete octet, and there is one additional bond drawn (resonance rules #1 and #2 both apply). This system can be thought of as four parallel 2p orbitals (one each on C2, C3, and C4, plus one on oxygen) sharing four pi electrons. One lone pair on the oxygen is in an unhybridized 2p orbital and is part of the conjugated pi system, and the other is located in an sp2 orbital.
Also note that one additional contributor can be drawn, but it is also minor because it has a carbon with an incomplete octet:
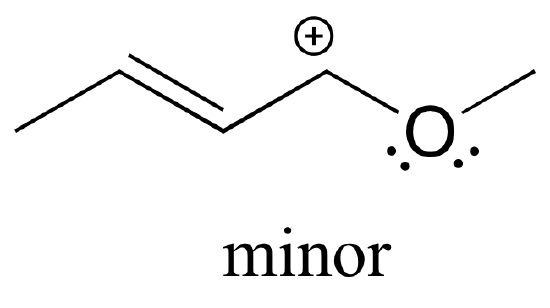
Exercise 2.6.5:
a) Draw three additional resonance contributors for the carbocation below. Include in your figure the appropriate curved arrows showing how one contributor is converted to the next.

b) Fill in the blanks: the conjugated pi system in this carbocation is composed of ______ 2p orbitals sharing ________ delocalized pi electrons.
Exercise 2.6.6: Draw the major resonance contributor for each of the anions below.
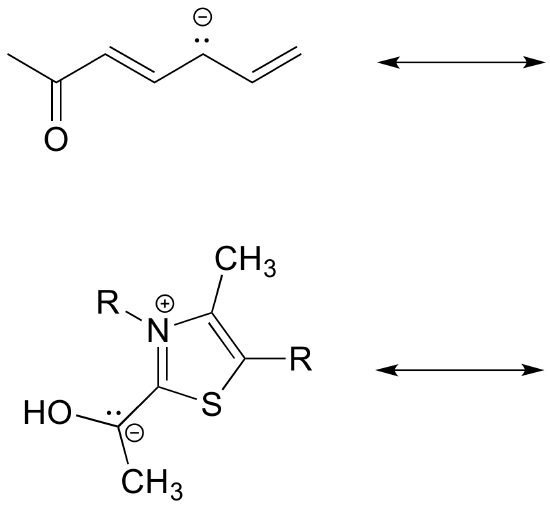
c) Fill in the blanks: the conjugated pi system in part (a) is composed of ______ 2p orbitals containing ________ delocalized pi electrons.
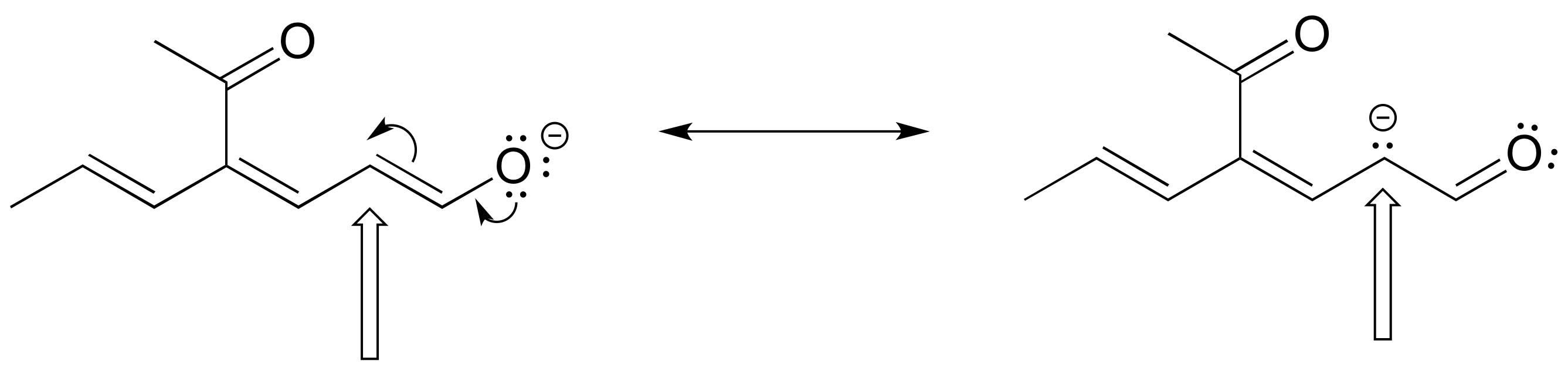
Exercise 2.6.7: The figure below shows how the negative formal charge on the oxygen can be delocalized to the carbon indicated by an arrow. More resonance contributors can be drawn in which negative charge is delocalized to three other atoms on the molecule.
a) Circle these atoms.
b) Draw the two most important resonance contributors for the molecule.
A word of advice
Becoming adept at drawing resonance contributors, using the curved arrow notation to show how one contributor can be converted to another, and understanding the concepts of conjugation and resonance delocalization are some of the most challenging but also most important jobs that you will have as a beginning student of organic chemistry. If you work hard now to gain a firm grasp of these ideas, you will have come a long way toward understanding much of what follows in your organic chemistry course. Conversely, if you fail to come to grips with these concepts now, a lot of what you see later in the course will seem like a bunch of mysterious and incomprehensible lines, dots, and arrows, and you will be in for a rough ride, to say the least. More so than many other topics in organic chemistry, understanding bonding, conjugation, and resonance is something that most students really need to work on 'in person' with an instructor or tutor, preferably using a molecular modeling kit. Keep working problems, keep asking questions, and keep at it until it all makes sense!
References
- Petrucci, Ralph H., et al. General Chemistry: Principles and Modern Applications. New Jersey: Pearson Prentice Hall, 2007. Print.
- Ahmad, Wan-Yaacob and Zakaria, Mat B. "Drawing Lewis Structures from Lewis Symbols: A Direct Electron Pairing Approach." Journal of Chemical Education: Journal 77.3: n. pag. Web. March 2000. Link to this journal: http://pkukmweb.ukm.my/~mbz/c_penerb...83%29/p329.pdf
Outside links
- http://en.wikipedia.org/wiki/Resonance_(chemistry)
- http://www.absoluteastronomy.com/topics/Resonance_(chemistry)#encyclopedia
- http://www.nku.edu/~russellk/tutorial/reson/resonance.html
- http://en.wikipedia.org/wiki/Formal_charge
- http://commons.wikimedia.org/wiki/Main_Page (for the electronegatvity chart)
- http://misterguch.brinkster.net/PRA037.pdf (for problem 5)
- http://commons.wikimedia.org/wiki/File:Phosphite-ion-resonance-structures-2D.png (for the (HPO32-) problem 4 answer)
- http://commons.wikimedia.org/wiki/File:Sulfate-resonance-2D.png (for the Sulfate answer)
- http://www.mpcfaculty.net/mark_bishop/resonance.htm
- http://www.chem.ucla.edu/harding/tutorials/resonance/draw_res_str.html
Problems
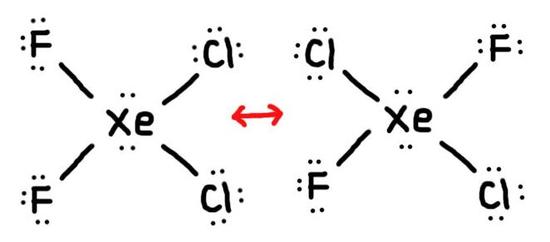
- True or False, The picture below is a resonance structure?
- Draw the Lewis Dot Structure for SO42- and all possible resonance structures. Which of the following resonance structure is not favored among the Lewis Structures? Explain why. Assign Formal Charges.
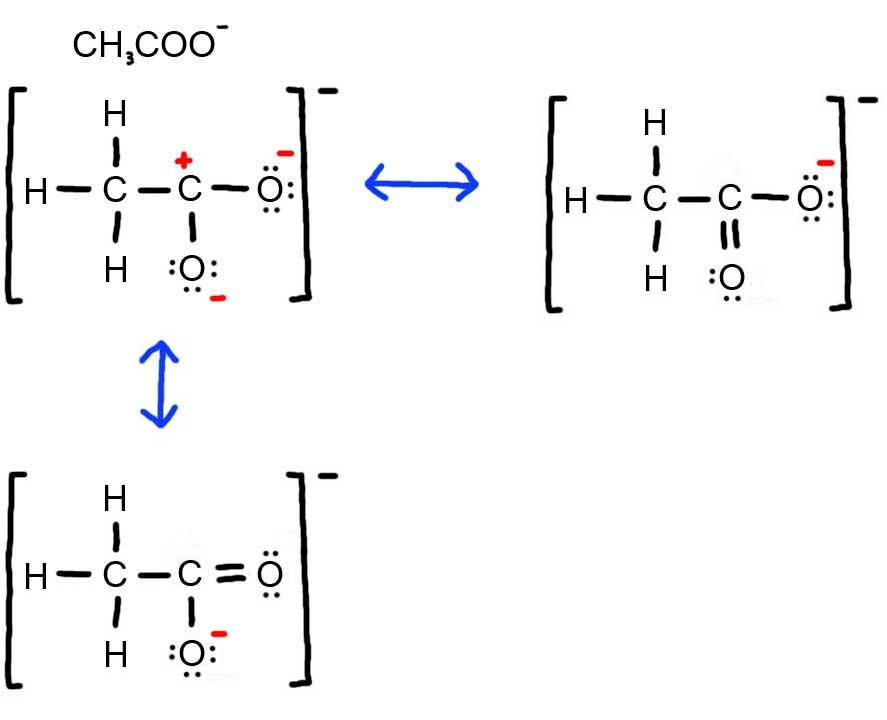
- Draw the Lewis Dot Structure for CH3COO- and all possible resonance structures. Assign Formal Charges. Choose the most favorable Lewis Structure.
- Draw the Lewis Dot Structure for HPO32- and all possible resonance structures. Assign Formal Charges.
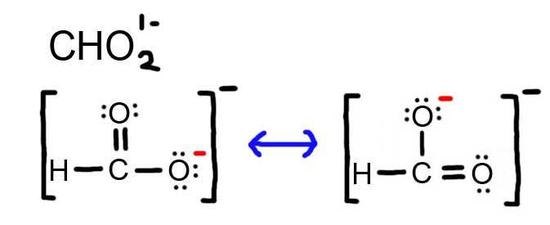
- Draw the Lewis Dot Structure for CHO21- and all possible resonance structures. Assign Formal Charges.
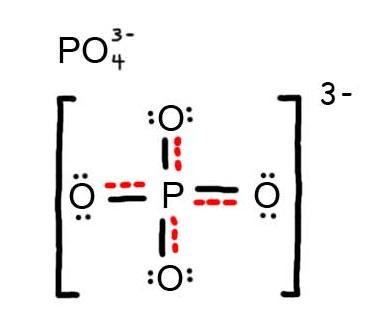
- Draw the Resonance Hybrid Structure for PO43-.
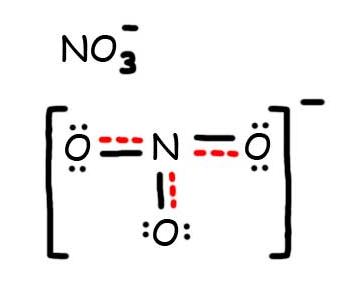
- Draw the Resonance Hybrid Structure for NO3-.
Answers
1. False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules).
2. Below are the all Lewis dot structure with formal charges (in red) for Sulfate (SO42-). There isn't a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity between the Oxygen atoms.
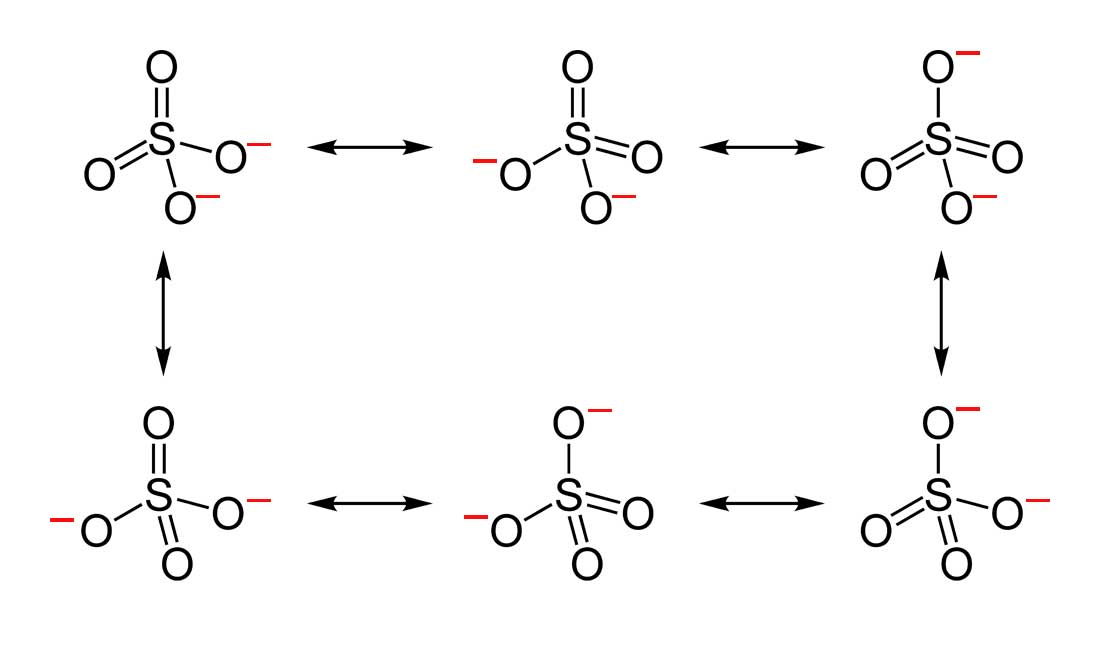
3. Below is the resonance for CH3COO-, formal charges are displayed in red. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge.
4. The resonance for HPO32-, and the formal charges (in red).
5. The resonance for CHO21-, and the formal charges (in red).
6. The resonance hybrid for PO43-, hybrid bonds are in red.
7. The resonance hybrid for NO3-, hybrid bonds are in red.
Contributors
- Sharon Wei (UCD), Liza Chu (UCD)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
- Layne Morsch (University of Illinois Springfield)
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Kelly Matthews, Harrisburg Area Community College