7.1.2: Summary of the three most common geometries
- Page ID
- 206984
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
A brief summary of the factors for predicting geometry
The three most important factors for determining metal complex geometry in biological metal binding sites:
- Stabilization Energy (including LFSE)
- Steric interactions between ligands
- Protein folding or constraints caused by limitations in bond angle (geometric constraints)
Splitting of d-orbitals in the three most-common geometries:
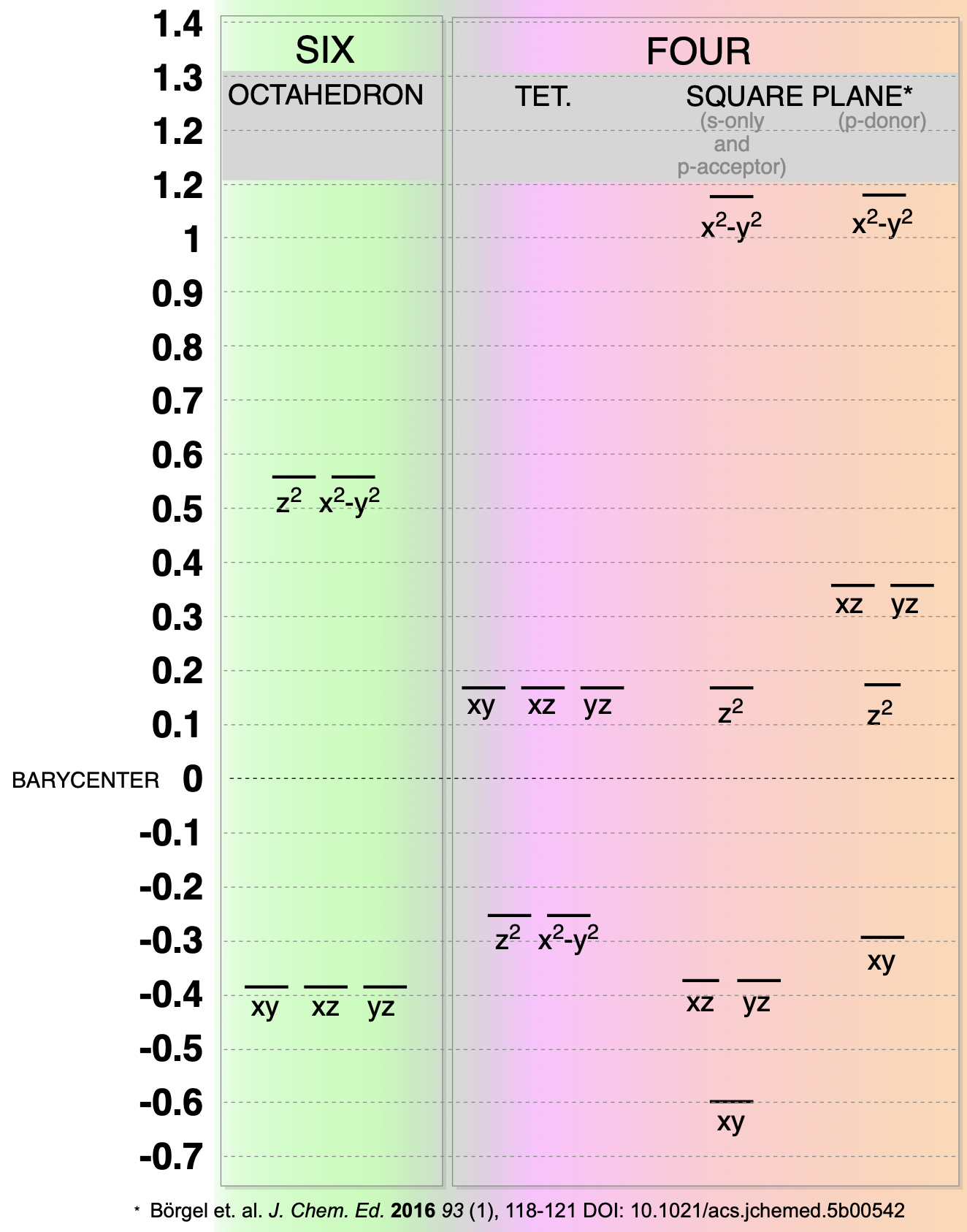
OCTAHEDRAL (most common)
- Is the preferred geometry for most metals because 6 ligands contribute to stabilizing electrophilic metal center.
- Will always have more negative (stable) LFSE than analogous tetrahedral case.
- Is more sterically crowded
Tetrahedral (2nd most common)
- Small \(\Delta\) means less negative LFSE (less stable in terms of LFSE)
- Always high spin
- Best case in terms of steric crowding around the metal center
Square Plane (\(d^8\) and \(d^9\))
- The bigger the \(\Delta\), the more likely it will be square planar due to huge LFSE benefit.
- Pt and Pd are almost always square planar while Ni is often tetrahedral.
- In the case of Ni, strong filed ligands favor square plane.
- \(d^9\) metals prefer square plane (or something similar) as a result of Jahn-Teller distortion of octahedral geometry.
- Highest-energy orbital is usually empty in \(d^8\).
Attribution
Curated or created by Kathryn Haas

