Exercise \(\PageIndex{1}\)
The laboratory of Samuel Stupp at Northwestern designs molecules with potential biomedical applications. The molecule below helps recruit growth factor to the cell (Christina J. Newcomb, Shantanu Sur, Sungsoo S. Lee, Jeong Min Yu, Yan Zhou, Malcolm L. Snead, and Samuel I. Stupp, Nano. Lett. 2016, 16, 3042-3050. Copyright 2016 American Chemical Society).
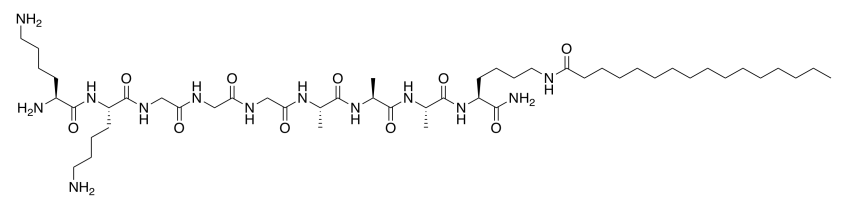
- This molecule is a synthetic amphiphile. Put a rectangle around the polar part of this molecule. Circle the nonpolar part of the molecule.
- The amphiphile inserts into the cell membrane. Draw a cartoon of a portion of a cell membrane. Add a cartoon of the synthetic amphiphile to the membrane. What is the IMF through which it interacts with the membrane?
- How would the amphiphile attract / bind growth factor and other proteins?
- This effect might be amplified if several of the amphiphiles bind to the membrane and cluster together. Use an abbreviated drawing (not the whole molecule) to show how two synthetic amphiphiles would interact with each other.
- This drawing is reminiscent of a specific structural type seen in proteins. Which one?
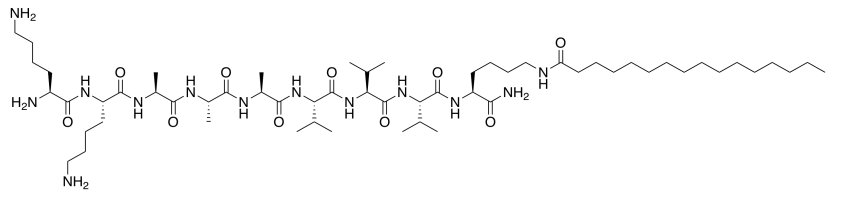
- The first amphiphile is not very good at interacting with others because it tends to spiral into a corkscrew shape. The amphiphile below is better at interacting with others because it keeps a flatter shape. Propose a reason why.
Exercise \(\PageIndex{2}\)
Here is a second type of amphiphile from the Stupp lab. This molecule was designed to bind a DNA strand (Adapted with permission from Mustafo Guler, Jonathan K. Pokorski, Daniel H. Appella, and Samuel I. Stupp, Bioconjugate Chem.. 2005, 16, 501-503. Copyright 2018 American Chemical Society).
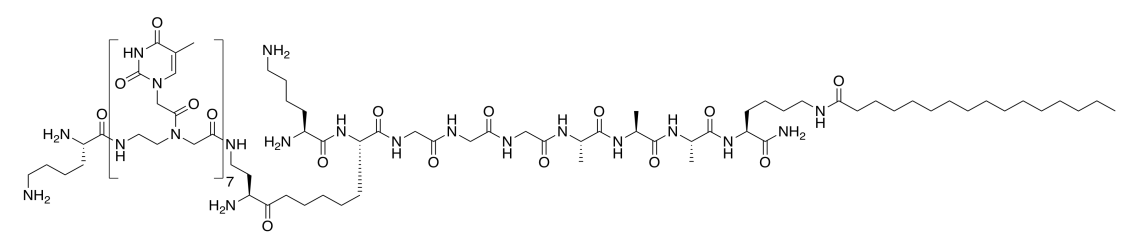
- Circle the part of the molecule that would bind to a DNA base.
- The base in the amphiphile binds to another DNA base. Draw the three other options.
- Which DNA base would the amphiphile bind best? Show how with a drawing.
- The melting point of DNA increases by 2°C for every AT pair; 4°C for every CG pair. Assuming all of the bases in the amphiphile bind to DNA, predict the melting point of the adduct.
- DNA actually binds more tightly to this amphiphile than to another DNA strand. Propose a reason why.
Exercise \(\PageIndex{3}\)
Problem IB5.3.
Liposomes are artificially prepared vesicles made of lipid bilayers. A drawing of a liposome is shown below along with its structural relative, a micelle.
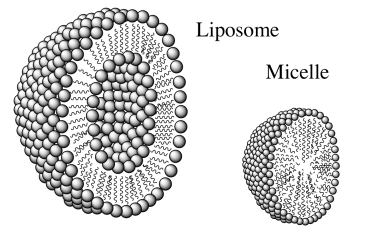
- Compare and contrast a liposome with a micelle.
- What type of molecules would be contained in the center of a liposome?
- What type of molecules would be contained in the center of a micelle?
- Liposomes are used for drug delivery due to their unique properties. A liposome encapsulates a drug that cannot easily pass through the lipid bilayer.
- A liposome can carry both hydrophobic molecules and/or hydrophilic molecules:
- [ Hydrophobic / Hydrophilic ] chemicals can be dissolved into the membrane. (Choose one.)
- [ Hydrophobic / Hydrophilic ] chemicals can be dissolved into the liposome's center. (Choose one.)
Another interesting property of liposomes is their ability to target cancer. Tumors do not have tight junctions between cells and are leaky. Liposomes of certain sizes, typically less than 200 nm, can rapidly enter tumor sites from the blood, but are kept out of healthy tissue by the endothelial wall. Anti-cancer drugs such as Doxorubicin and Camptothecin (shown below) are currently being marketed in liposome delivery systems.
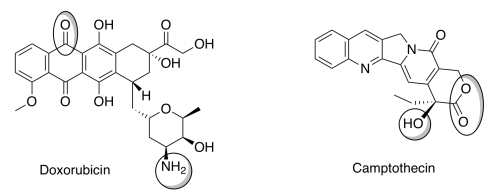
f) Identify each of the circled functional groups.
g) To encapsulate these drugs in liposomes, you will need to predict whether they are water soluble or not. Choose the best attribute for each compound.
Doxorubicin [water-soluble / insoluble in water]
Camptothecin [water-soluble / insoluble in water]
To deliver the molecules to sites of action, the lipid bilayer can fuse with other bilayers such as a cell membrane, thus delivering the liposome contents.
Since cholesterol can improve the stability of lipid bilayers, some researchers designed and synthesized a category of phospholipids that incorporate cholesterol covalently linked to the glycerol backbone of phosphatidylcholine (shown below).
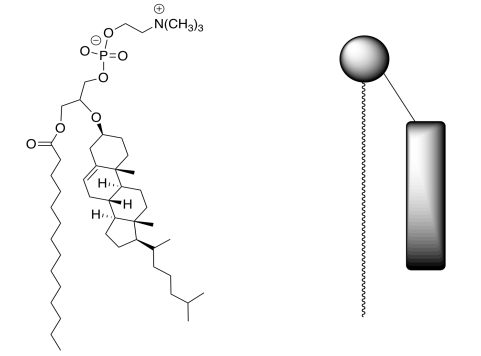
h) On the structure of the phosphatidylcholine, put a circle around the polar head, a rectangle around the cholesterol group, and a dashed ellipse around the fatty acid group.
i) Draw a picture of the lipid bilayer formed with this phospholipid (use the cartoon on the right) vs a regular phospholipid with cholesterol added in.
Data on animal models suggest that liposomes formed with the sterol modified phospholipid are very effective for delivery of anti-cancer agents.
j) Suggest at least two advantages to the sterol modified liposomes.







