Haemocyanin
- Page ID
- 194800
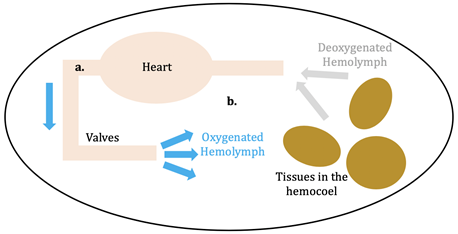
Hemocyanin (Hc; plural: Hcs) is the oxygen transporter protein present in the hemolymph of arthropods (eg: horseshoe crabs, arachnids) and molluscs (eg: octopi).1 Hemolymph is the circulating fluid for these animals that is the invertebrate equivalent to vertebrate blood.2 This circulatory fluid exists in the open circulatory system of the invertebrates, whereas blood is within the vertebrate’s closed circulatory system. In an open circulatory system, hemolymph is confined to vessels for only a portion of its journey through the body (see figure 1, a); the rest of the time it is within hemocoel, or the main body cavity, of the invertebrate (see figure 1, b). After the hemolymph leaves the valves surrounding the heart, it is directly put into the hemocoel to oxygenate the tissues.3 In closed circulatory systems, on the other hand, blood is pumped in a closed circuit of veins and arteries to reach tissues in the body.
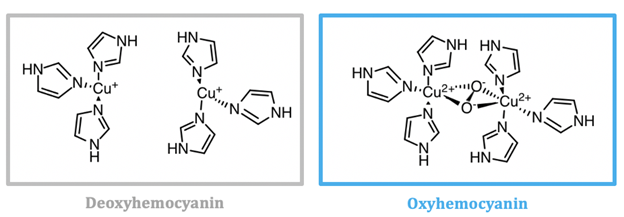
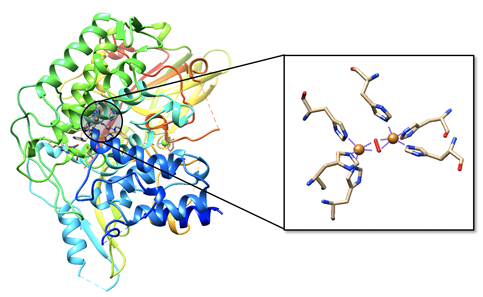
Mollusc and arthropod Hcs are structurally very different, however both contain two coppers at the center coordinated to three histidines, and both are used for transport of molecular oxygen in hemolymph. Arthropod Hc have kidney-shaped subunits, each with an oxygen binding site, arranged into hexamers. Mollusc Hc, on the other hand, is composed of about 10 subunits forming a hollow cylinder. Recently, there has been research into different therapeutic applications of hemocyanins. Studies suggest these metalloproteins have applications as viral and bacterial antigens, immune-stimulants for treatment of some cancers such as melanoma, and carrier molecules for vaccines.9
Selectivity
Hard/Soft Acid/Base Theory (HSAB)
Hard/Soft Acid/Base (HSAB) Theory allows for predictions of which acids and bases (metals and ligands) prefer to interact and which are most stable. In HSAB Theory, acids are electron acceptors and are referred to as acids/metal ions, and bases are electron donors and are referred to as ligands. These acids and bases can be categorized as hard or soft based on their charge density (charge to size ratio), polarizability (ability for an induced dipole to occur), and the covalent vs ionic nature of their interactions. Hard acids and bases have a higher charge density, are less polarizable, and/or have bonds that are more ionic; these tend to have a high charge and a small radius. On the other hand, soft acids and bases have a lower charge density, are more polarizable, and/or they have bonds that are more covalent; these tend to have a low charge and a large radius. HSAB can be used to predict which different metal-ligand pairs prefer to interact; hard acids prefer to coordinate with hard bases, soft acids prefer to coordinate with soft bases, and borderline acids prefer borderline bases. Essentially, the more “like” the characteristics of a metal and ligand, the stronger the preference of the ligand for that metal. This means that if there is a soft acid present, \(\ce{Au^{+}}\) for example, in the active site of a molecule, it will prefer to bind to a soft base, such as \(\ce{S^{2-}}\).10 In general, when you move down the periodic table, softness increases, and as you move right softness increases. Additionally, all of the bases are nonmetal electron donors (typically anions), and the acids are mostly metal ions.
As seen in figure 2, the active site of hemocyanin contains two copper ions, each bound to three histidine (His) residues. When the Cu(I) ion binds to oxygen it is oxidized to Cu(II). Cu(I) is a soft acid, Cu(II) is a borderline acid, and the imidazole ring of histidine is a borderline base. Atoms or molecules that are classified as having intermediate characteristics are called borderline, as they do not lie at either extreme of the hard/soft spectrum; they are harder than metals that are categorized as “soft” and softer than metals categorized as “hard”. Nitrogens are typically classified as hard bases, however if a nitrogen is contained within an aromatic ring, the entire ring is more polarizable. For this reason, imidazole nitrogen atoms are classified as borderline bases. Because the copper ion alternates between a soft acid (Cu(I)) and a borderline acid (Cu(II)), it makes sense that it is bound to a borderline base. Borderline histidine is a better match for borderline Cu(II) than soft Cu(I), however His is still a better match for Cu(I) than a set of ligands that is harder. It is important to recognize, however, that there are other factors that affect metal ion selectivity, such as ionic size and ligand field stabilization energy, that must also be considered.
Ionic Size
Hemocyanin exists in the hemolymph of invertebrates. Hemolymph is simply the invertebrate equivalent of blood that is present in vertebrates and is composed of water and organic and inorganic compounds. The inorganic metals present in hemolymph are sodium, chloride, calcium, magnesium, potassium, and manganese ions.11,12 \(\ce{Na^{+}}\), \(\ce{Ca^{2+}}\), \(\ce{Mg^{2+}}\), \(\ce{K^{+}}\), and \(\ce{Mn^{2+}}\) can all be seen as potential competitors for the copper at the active site due to the fact that they are all positively charged metal ions. They are all hard acids, indicating that they will likely not bind well with histidine, a borderline base. Based solely on HSAB, the histinines will prefer to bind to Cu(I)/Cu(II) because they are either soft/borderline acids. In addition to HSAB theory, it is also important to consider ionic size when determining if these competitors will bind in the site. These metal ions are larger than the copper ions, which may affect their ability to bind to the histidines in the pre-organized hemocyanin active site; the larger ions will not fit well into the pre-organized site, or if the site could accommodate the larger ion, it may change the protein structure to cause unfavorable steric interactions. While ionic size is often an important consideration when looking at competitors of a metal ion, it likely does not matter much here. The unfavorable HSAB interactions that would occur if \(\ce{Na^{+}}\), \(\ce{Ca^{2+}}\), \(\ce{Mg^{2+}}\), \(\ce{K^{+}}\), or \(\ce{Mn^{2+}}\) replaced the copper ion are sufficient enough to say that these ions would not bind here, regardless of their size.
D-orbital splitting
Ligand field stabilization energy (LFSE) refers to the increased stabilization of a complex that occurs due to splitting of the d-orbitals. Rather than having 5 degenerate (same energy) orbitals, the orbitals split so that some are lowered in energy and others are raised in energy. In oxyhemocyanin, Cu(II) is found in a distorted tetrahedral geometry.12 Typically d9 metal complexes, such as Cu(II), prefer to be in a square planar environment, rather than tetrahedral.14 This is because more electrons can be placed in lower-energy orbitals in a square plane compared to a tetrahedron. In figure 4, it can be noted that four of the five orbitals for the square planar geometry are at a lower energy level relative to the tetrahedral geometry. There is only one orbital at a high energy level, so it is more energetically favorable to have 8 electrons at the lower level and one electron at the high level. In the tetrahedron, on the other hand, there are five electrons at a relatively high energy level, so overall there is less LFSE. This explains why d9 metals such as Cu(II) prefer to be in a square planar ligand field. While many d9 metal complexes prefer the square planar environment, there is a significant number of d9 metals that have structures that display a structure that is intermediate between a square plane and tetrahedron. This type of intermediate structure is the case with Cu(II) in oxyhemocyanin.13,14
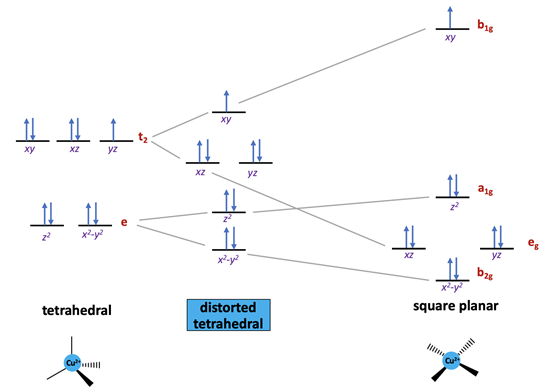
When there is an uneven occupation of the t2 orbitals in the tetrahedral geometry, it gives rise to Jahn-Teller (JT) distortion. JT distortion occurs due to unequally distributed electrons in degenerate orbitals. Typically, the distortion occurs in a way that places partially-filled orbitals at a slightly higher energy, allowing the fully-filled degenerate orbitals to be lowered in energy. This occurs to increase the overall stability of the molecule. d9 metals with a lower occupation in one of the t2 orbitals have been shown to distort from a tetrahedron toward a square plane.14 This expected Jahn-Teller distortion of a tetrahedron is consistent with the appearance of a distorted tetrahedral geometry for Cu(II) bound to hemocyanin. As seen in the t2 antibonding orbital of the tetrahedral arrangement in figure 4, there are two pairs of electrons that are paired, and one that is unpaired. The unpaired electron shifts slightly up in energy so that the other two degenerate orbitals will be lowered in energy, increasing the overall stability of the complex.
Although it makes sense for Cu(II) to appear in a distorted geometry, Cu(II) in hemocyanin is not in the most favorable geometry according to ligand field theory (LFT) arguments; the square plane is preferred. To rationalize the pseudo-tetrahedral geometry, we should also consider other factors, especially steric or conformational restrains imposed by protein folding. The stable protein fold of hemocyanin is likely to create a pre-organized binding site so that Cu(II) is forced to bind in this way. The ligands at hemocyanin’s active site are ‘locked’ in place so that Cu(II) is forced to be in a tetrahedral environment. In order to make the tetrahedral environment more energetically favorable, the geometry is slightly distorted by the JT effect toward the more energetically favorable square plane.
When in the deoxygenated form, Cu(I) ions are coordinated to three histidine residues in a trigonal pyramidal geometry. Cu(I) is coordinatively unsaturated because it is only bound to three ligands, leaving one open coordination site on each copper ion that can accept an additional ligand; that ligand is \(\ce{O_{2}}\). Additionally, because Cu(I) is a d10 metal, there is no additional LFSE. A d10 metal is one that has all 10 electrons in the outer d-orbital. This means that Cu(I) has no geometric preference based on LFSE. If sterics were the major factor affecting the Cu(I) complex geometry, a three-coordinate metal center would prefer to be in a trigonal planar environment because it has the largest bond angles (120°) and therefore the least steric strain. In this case, however, it is bound in a trigonal pyramidal geometry (bond angles = 109.5°) because the protein binding site is prearranged. It is important that Cu(I) must be coordinated in a trigonal pyramidal geometry where it is not completely stable because that allows it to accept oxygen for transport. If Cu(I) was in the trigonal planar geometry (which is preferred based on sterics), it might be so stable that it will not easily oxidized to Cu(II).
Cu(I) and Cu(II) are both found in geometries that are not typically preferred; if the copper ion is too stable in a certain geometry, then it will become more difficult to change oxidation state. The function of hemocyanin is oxygen transport, so it is vital that it can bind and release oxygen quickly, changing oxidation states as it does so. If Cu(II) was in the preferred square planar geometry, reduction to Cu(I) and release of oxygen would be less favorable. Similarly, if Cu(I) were in a geometry that was strongly disfavored by Cu(II), oxidation would be less favorable, so it would be more difficult to bind and transport oxygen.
Another important consideration are the metal-ligand interactions. As previously mentioned, copper is bound to three histidine residues. These histidine residues contain imidazole rings, which are \(\sigma\)-donors and weak \(\pi\)-acceptors. \(\sigma\)-donors are ligands with one lone pair of electrons, and \(\pi\)-acceptors are ligands contain a \(\pi\) bond, and subsequently an unoccupied \(\pi\)* orbital. In the spectrochemical series, imidazole rings lie between pyridine amines and \(\ce{NH_{3}}\). In order of increasing basicity and \(\pi\) electron acceptor capability, pyridine < imidazole < \(\ce{NH_{3}}\).15 As the basicity of the ligand increases, the splitting distance of the metal’s d orbital, \(\Delta\), increases. However, because histidine ligands are weak \(\pi\)-acceptors, they are considered relatively weak field ligands that do not have a significant effect on \(\Delta\). In figure 4, \(\Delta\) refers to the distance between the a1g and b1g orbitals.
Color of the Complex
Upon oxygen binding, Cu(I) is oxidized to Cu(II) and the complex shifts from colorless to blue. The number of electrons in the d-orbital, as well as phenomena of d-orbital splitting explains why deoxyhemocyanin with Cu(I) at the center is colorless, whereas oxyhemocyanin with Cu(II) at the center is blue. The vivid colors exhibited by transition metal complexes are caused by the d-d and charge transfer transitions when an electron is excited from a lower energy d-orbital to a higher energy d-orbital within the visible range of light. Cu(I) is a d10 metal, so its valence d-orbital is full; therefore, electrons in the lower energy d-orbitals cannot be excited to the higher energy d-orbitals. Charge-transfer transitions can occur with Cu(I) ions, however they are rarely observed due to the high energy required outside of the UV-vis range to excite the electrons. These types of transitions occur between molecular orbitals that are mostly metal in character and those that are mostly ligand in character. Specifically, metal to ligand charge transfer (MLCT) transitions can occur in this case because Cu(I) is bound to the imidazole rings of histidine residues, which are \(\pi\) accepting ligands.
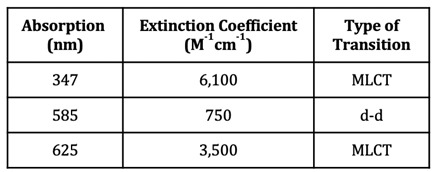
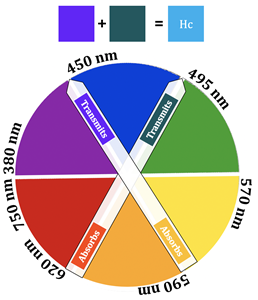
In the case of Cu(II), however, there is a partially filled orbital at a higher energy level that electrons can be excited to. The oxyhemocyanin complex has wavelengths of maximum absorbance at 347 nm, 585 nm, and 625 nm. The corresponding extinction coefficients of these wavelengths of absorption can be found in table 1. Extinction coefficients that are greater than 1,000 are typical of charge transfer transitions, while extinction coefficients less than 1,000 are typical of d-d transitions. Additionally, larger extinction coefficients indicate higher intensity absorbances. Based on the extinction coefficients, the d-d transition for oxyhemocyanin occurs at 585 nm, whereas the MLCT transitions occur at 347 nm and 625 nm. The absorbance at 347 nm does not contribute to the color of the complex because 347 nm is outside of the visible region of light (~400-800 nm). A combination of the 585 nm and 625 nm absorbances explain the blue color of the oxyhemocyanin complex. The extinction coefficient at 625 nm is about 4.7x greater than the extinction coefficient at 585 nm, indicating that the absorbance at 625 nm is stronger than the one at 585 nm. This means that the 625 nm absorption contributes most to the color of the complex, so the complex absorbs yellow/orange light, therefore transmitting blue/green light (the color opposite orange on the color wheel) (see figure 5).16,17 The absorbance that occurs at 585 nm has a slight contribution to the color of the complex as well; this color corresponds to orange light, so the complex transmits blue light. These two absorbances are what account for the blue color of the complex.
Kinetics and Thermodynamics
The stability of the complex is influenced by the energy of electrons in the d-orbital, as well as the geometry of the metal complexes. Because Cu(I) and Cu(II) have electrons at a higher energy, it makes them less stable and more likely to react. Additionally, Cu(II) is coordinated in a distorted tetrahedral geometry, but based on LFT, the square planar geometry is preferred. If Cu(II) was in the preferred geometry it would be more stable, and therefore reduction to Cu(I) and release of oxygen would be less favorable. Because Cu(II) is not fully stable in this environment, it allows for the cycling between the +1 and +2 oxidation states, and the transport of oxygen.
Lability refers to the rate that ligands associate to/dissociate from metal ions. Hemocyanin is known to be labile, which means oxygen can bind and unbind quickly to/from the copper center.18 Cu(I) is a d10 metal and Cu(II) is a d9 metal, so their higher-energy antibonding t2 orbitals are occupied by electrons. When electrons occupy this higher-energy set of d-orbitals, the complex is more kinetically labile (has fast substitutions reactions). Given the function of hemocyanin, it makes sense that it is labile; oxygen needs to be able to quickly dissociate to oxygenate tissues.
Redox
Hemocyanin has a copper ion at the active site that cycles between Cu(I) and Cu(II) depending on whether or not oxygen is bound. Cu(I) is oxidized by oxygen when \(\ce{O_{2}}\) coordinates with the metal. In this case, oxygen is the oxidizing agent because it accepts the extra electron from Cu(I); Cu(I) is the reducing agent because it donates an electron to \(\ce{O_{2}}\). For the copper oxidation half reaction, the reduction potential at pH = 7 is 0.153 V.19 The one electron reduction of \(\ce{O_{2}}\) cannot occur because it is spin forbidden.20 The reduction potential of this reaction at pH = 7 is -0.33 V, which makes the overall reaction potential less than 0.19 Reaction potentials less than 0 are unfavorable and the reaction is not spontaneous.
In reality, there are two electrons and two hydrogen ions involved in the reduction of \(\ce{O_{2}}\) to \(\ce{O_{2}^{2-}}\); the reduction potential of this reaction at pH = 7 is 0.281 V, which makes the overall reaction potential greater than 0, showing that the reaction is favorable.5 This means that the overall redox potential of the reaction is 0.128 V. Since the value is greater than 0, the reaction is favorable, indicating that the binding of oxygen to hemocyanin is spontaneous. The oxidation and reduction half reactions can be seen below, as well as the overall reaction for the system.
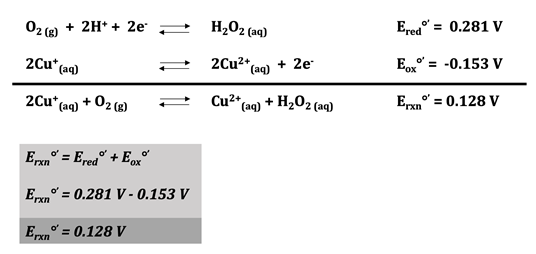
Figure 6.19 Oxidation-Reduction Reactions for Hemocyanin. The reduction half reaction can be seen on the first line. There are two electrons and two hydrogen ions involved in the reduction of oxygen, and the reduction potential of this reaction is 0.281 V at pH 7. On the following line, the oxidation half reaction can be seen where Cu(I) is oxidized to Cu(II) with an oxidation potential of -0.153 V at pH 7. The overall reaction appears on the third line. Redox potential of the overall reaction is calculated by adding the reduction and oxidation potentials together. At pH 7, the redox potential of this reaction is 0.128 V. Because this is greater than zero, the overall reaction is favorable and the binding of oxygen to hemocyanin is spontaneous.
Conclusion
Hemocyanin is the oxygen transporter protein in molluscs and arthropods. Oxygen binds to a dicopper center at the protein’s active site. When oxygen binds, Cu(I) is oxidized to Cu(II), causing a color change of the complex from colorless to blue. The oxidation of Cu(I) and reduction of O2 is spontaneous, with an Erxn°’ of 0.128 V. Deoxyhemocyanin does not absorb in the visible region, explaining why it appears colorless, and oxyhemocyanin absorbs at 347 nm, 585 nm, and 625 nm. 347 nm is outside of the visible region of light, however 585 nm and 625 nm are not; these two colors are associated with red/orange light, meaning that the complex transmits blue light, thus explaining why it appears blue. Cu(I), a d10 metal, is coordinated to deoxyhemocyanin in a trigonal pyramidal geometry, whereas Cu(II), a d9 metal, is coordinated to oxyhemocyanin in a distorted tetrahedral geometry. Hard/soft acid/base theory explains why both Cu(I), a soft acid, and Cu(II), a borderline acid, can both coordinate to histidine, a borderline base. Borderline histidine is a better match for borderline Cu(II) than soft Cu(I), however His is still a better match for Cu(I) than a set of ligands that is harder.
Sources
- Cook, J. D.; Penner‐Hahn, J. E.; Stemmler, T. L. Structure and Dynamics of Metalloproteins in Live Cells. Methods in Cell Biology Methods in Nano Cell Biology 2008, 199–216.
- Kanost, M. R. Hemolymph. Encyclopedia of Insects 2009, 446–449.
- Christian, W. S.; Stefan, R. Functional morphology and diversity, Chapter 14: Circulatory System and Respiration; Oxford University Press: Oxford, 2013.
- Hashim, O. H.; Adnan, N. A. Coenzyme, Cofactor and Prosthetic Group — Ambiguous Biochemical Jargon. Biochemical Education 1994, 22 (2), 93–94.
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Morooka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, K.; Tatsumi, K.; Nakamura, A. A New Model for Dioxygen Binding in Hemocyanin. Synthesis, Characterization, and Molecular Structure of the .Mu.-.Eta.2:.Eta.2 Peroxo Dinuclear Copper(II) Complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = Isopropyl and Ph). Journal of the American Chemical Society 1992, 114 (4), 1277–1291.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- Hazes, B.; Hol, W. Oxygenated Hemocyanin (Subunit Type Ii). 1996.
- Coates, C. J.; Decker, H. Immunological Properties of Oxygen-Transport Proteins: Hemoglobin, Hemocyanin and Hemerythrin. Cellular and Molecular Life Sciences 2016, 74 (2), 293–317.
- Libretexts. 3.2.1: Hard and Soft Acid and Base Theory. https://chem.libretexts.org/Courses/Saint_Mary's_College,_Notre_Dame,_IN/CHEM_342:_Bio-inorganic_Chemistry/Readings/Week_3:_Metal-Ligand_Interactions_continued..../3.2:_The_identity_of_metal_ion_and_the_ligand_donor_atom(s)_affects_affinity/3.2.1:_Hard_and_Soft_Acid_and_Base_Theory (accessed Jan 28, 2020).
- Pratoomchat, B.; Sawangwong, P.; Pakkong, P.; Machado, J. Organic and Inorganic Compound Variations in Haemolymph, Epidermal Tissue and Cuticle over the Molt Cycle in Scylla Serrata ž Decapoda/. 2002, 13.
- Sowers, A. D.; Young, S. P.; Grosell, M.; Browdy, C. L.; Tomasso, J. R. Hemolymph Osmolality and Cation Concentrations in Litopenaeus Vannamei during Exposure to Artificial Sea Salt or a Mixed-Ion Solution: Relationship to Potassium Flux. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2006, 145 (2), 176–180. https://doi.org/10.1016/j.cbpa.2006.06.008.
- Morris, R. H.; Lee, A.; Hadzovic, A. A Tour of Hemocyanin. https://sites.chem.utoronto.ca/chemistry/coursenotes/GTM/JM/hemocyanin/start.htm (accessed Mar 27, 2020).
- Cirera, J.; Ruiz, E.; Alvarez, S. Stereochemistry and Spin State in Four-Coordinate Transition Metal Compounds. Inorganic Chemistry 2008, 47 (7), 2871–2889.
- Sundberg, R. J.; Martin, R. B. Interactions of Histidine and Other Imidazole Derivatives with Transition Metal Ions in Chemical and Biological Systems. Chemical Reviews 1974, 74 (4), 471–517.
- Nickerson, K. W.; Holde, K. E. V. A Comparison of Molluscan and Arthropod Hemocyanin—I. Circular Dichroism and Absorption Spectra. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1971, 39 (4), 855–872.
- Frieden, E.; Osaki, S.; Kobayashi, H. Copper Proteins And Oxygen: Correlations Between Structure And Function Of The Copper Oxidases. Journal of General Physiology 1965.
- Tommerdahl, A. P.; Burnett, K. G.; Burnett, L. E. Respiratory Properties of Hemocyanin From Wild and Aquacultured Penaeid Shrimp and the Effects of Chronic Exposure to Hypoxia. The Biological Bulletin 2015, 228 (3), 242–252.
- Lippard, S. J.; Berg, J. M. Principles of bioinorganic chemistry; University Science Books: Mill Valley, CA, 1994.
- Solomon, E. I.; Heppner, D. E.; Johnston, E. M.; Ginsbach, J. W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M. T.; Kjaergaard, C. H.; Hadt, R. G.; Tian, L. Copper Active Sites in Biology. Chemical Reviews 2014, 114 (7), 3659–3853.
Contributed By:
Margaret Benjamin, a chemistry major at Saint Mary's College, class of 2020.

